Translate this page into:
Leukotrichia due to generic dasatinib
*Corresponding author: Ankur Jain, Department of Haematology, Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India. drankur589@yahoo.in
-
Received: ,
Accepted: ,
How to cite this article: Jain A, Chaudhry S. Leukotrichia due to generic dasatinib. J Hematol Allied Sci. 2023;3:164-5. doi: 10.25259/JHAS_29_2023
Dear Editor,
Dasatinib, a potent second-generation tyrosine kinase inhibitor (TKI), is approved for the frontline treatment of both the chronic and blast phases of chronic myeloid leukemia (CML). Inhibition of BCR::ABL accounts for the observed efficacy of dasatinib in CML. However, inhibition of other receptor kinases, namely Src family kinases, c-Kit, platelet-derived growth factor receptor (PDGFR), and ephrin A2 receptor, is responsible for its off-target adverse effects.[1] The use of dasatinib is mainly associated with hematological and cardiopulmonary toxicities. Cutaneous side effects of dasatinib are uncommon.[2]
A 42-year-old Indian lady was diagnosed with de novo myeloid blast crises of CML in June 2022. She denied acute myeloid leukemia-like intensive chemotherapy. Therefore, she was started on a combination of azacitidine and generic dasatinib (140 mg once daily per oral). Due to poor response after four months of therapy (BCR::ABL- 22.79%), the dasatinib dose was escalated to 180 mg once daily. Tyrosine kinase domain mutation analysis did not reveal any mutations. Two months later, she reported gradual whitening of scalp hair, eyebrows, and eyelashes [Figure 1]. Her complete blood count and systemic examination were unremarkable. A possibility of dasatinib-induced leukotrichia was considered. Due to an ongoing molecular response (6-month BCR::ABL-9.0%), dasatinib was continued. The patient was reassured about the cosmetic side-effect of dasatinib.
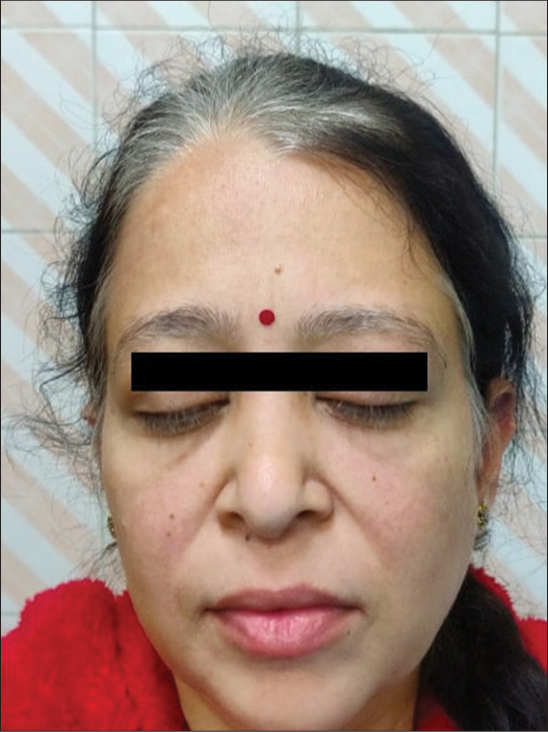
- Clinical photograph of a 42-year-old Indian lady with chronic myeloid leukemia in blast crises who was prescribed generic dasatinib 140 mg once daily. The image shows white hair, eyebrows, and eyelashes that developed 2 months after increasing the dasatinib dose to 180 mg once daily.
C-Kit, Src family kinases, and PDGFR play a pivotal role in all the phases of melanocyte development, including migration, proliferation, and maintenance. Inhibition of these receptors has been postulated as a possible mechanism for the cutaneous adverse effects seen with TKIs.[3] Among TKIs approved for the treatment of CML, cutaneous side effects are most commonly seen with imatinib. Imatinib use has been associated with both hypo- and hyperpigmentation of skin, hair, and nails.[4] Infrequent cutaneous toxicity of dasatinib could be explained by its lesser affinity for Src family kinases and c-Kit. Dasatinib-induced hypopigmentation has been described in only a few anecdotal case reports.[1] No case of leukotrichia due to generic dasatinib has been reported to date. Leukotrichia due to dasatinib is dose related and usually reversible upon drug discontinuation.[1] Given the availability of generic dasatinib in India, clinicians must be vigilant about this relatively rare side effect.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Dasatinib-induced hypopigmentation in pediatric patient with chronic myeloid leukemia: A case report and review of the literature. Case Rep Dermatol Med. 2018;2018:4062431.
- [CrossRef] [PubMed] [Google Scholar]
- A narrative review on adverse effects of dasatinib with a focus on pharmacotherapy of dasatinib-induced pulmonary toxicities. Blood Res. 2021;56:229-42.
- [CrossRef] [PubMed] [Google Scholar]
- Imatinib-induced generalized vitiligo. Br J Haematol. 2022;197:511.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term mucocutaneous adverse effects of imatinib in Indian chronic myeloid leukemia patients. Int J Dermatol. 2018;57:332-8.
- [CrossRef] [PubMed] [Google Scholar]






