Translate this page into:
Hidden amyloidosis in liver and bone marrow
*Corresponding author: Gayathri Jagadish, Department of Hematopathology, Sri Shankara Cancer Hospital and Research Center, Bengaluru, Karnataka, India. drgayathri39@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jagadish G, Archana M, Nataraj KS, Desai S, Kurpad V. Hidden amyloidosis in liver and bone marrow. J Hematol Allied Sci. 2023;3:155-7. doi: 10.25259/JHAS_45_2023
Abstract
Primary amyloidosis and multiple myeloma (MM) involve clonal plasma cell proliferation. Approximately 10–15% of patients with amyloidosis have MM. Characteristic apple-green birefringence using Congo red staining on polarized microscopy confirms amyloid. MM is prevalent at ages from 60 to 70 years. Amyloidosis commonly affects the heart, kidneys, gastrointestinal tract/liver and peripheral or autonomic nervous system predicting a poor prognosis due to organ deterioration. This case highlights the importance of diagnosing amyloidosis in a 60-year-old female where plasma cells were present in the liver and bone marrow along with amyloid deposits and for early initiation of intense chemotherapy.
Keywords
Multiple myeloma
Amyloidosis
Apple green birefringence
Intense chemotherapy
INTRODUCTION
Multiple myeloma (MM) is a hematological condition characterized by the abnormal proliferation of clonal, terminally differentiated B lymphocytes in the bone marrow.[1] Amyloidosis is a rare systemic disease where various low-molecular-weight protein subunits circulating in plasma form non-soluble fibrils in the form of beta-pleated sheets and are deposited in the extracellular tissue.[2]
CASE REPORT
A 60-year-old female presented to the surgical oncology department with abdominal fullness. Ultrasound revealed enlargement of the liver with multiple small suspicious lesions. A triple-phase computed tomography scan of the abdomen showed an enlarged hypodense liver with minimal enlargement of the spleen. Multiple small lytic lesions in the axial and appendicular skeleton with no pathologic fractures and sclerotic margins were noted [Figure 1a and b]. Serum alpha-fetoprotein (AFP) was 1.43 ng/mL (0–6.05). M band was seen on serum electrophoresis. Bone marrow aspiration showed 35–40% plasma cells with binucleate and trinucleate forms, suggesting plasma cell myeloma [Figure 2a and b]. Trephine biopsy showed increased plasma cells with homogenous eosinophilic material in the interstitium, raising suspicion of amyloid deposits [Figure 2c]. Conventional karyotyping and fluorescence in situ hybridization were non-contributory. A liver biopsy showed sheets of plasma cells with amyloid deposits [Figure 2d]. Congo red stain confirmed the amyloid on both liver and bone marrow biopsies [Figure 2e and f].
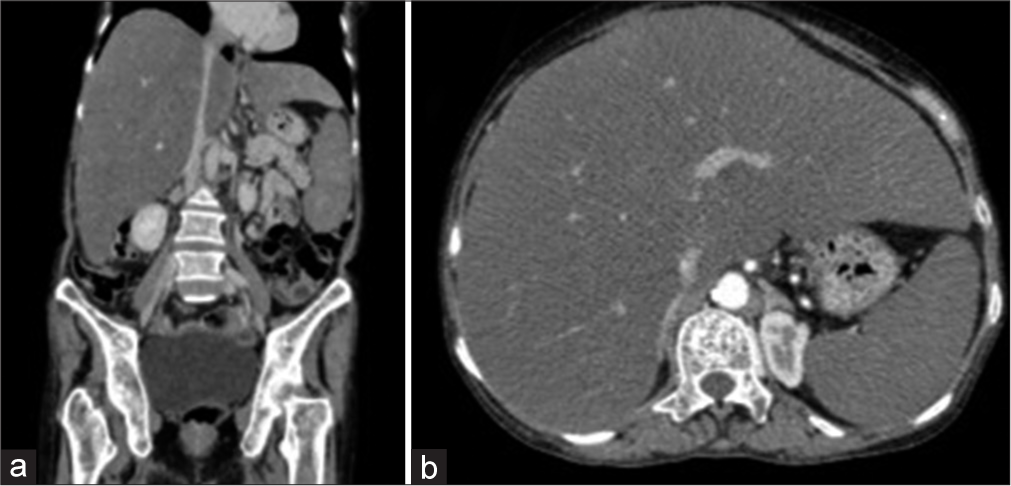
- (a and b) Computed tomography scan of the abdomen showing an enlarged hypodense liver with minimal enlargement of the spleen and multiple small lytic lesions in the axial and appendicular skeleton.

- (a and b) Bone marrow aspirate showing plasma cells. Binucleate and trinucleate forms are seen, (c and d) Trephine and liver biopsy showing increased plasma cells and homogenous eosinophilic material, (e and f) Congo red stain confirming the amyloid on both liver and bone marrow biopsies.
DISCUSSION
MM is a plasma cell dyscrasia that causes excessive monoclonal immunoglobulin production, chronic anemia, skeletal lesions, renal failure, and recurrent infections. Myeloma-associated kidney diseases include cast nephropathy, light chain amyloidosis (AL) amyloidosis, monoclonal immunoglobulin deposition disease, cryoglobulinemic glomerulonephritis, and proliferative glomerulonephritis.[3]
Kidneys are the most common organ involved, followed by the heart.[4] Approximately half of the systemic amyloidosis cases have even liver involvement.[5] In general, the presentation of hepatic amyloidosis varied with minimal or no symptoms. [6,7] The most common finding of hepatic amyloidosis is hepatomegaly (present in 80–90% of patients),[8] which was observed in our patient. Gertz and Kyle reported hepatomegaly in 16 (84%) of 19 patients with amyloidosis involving the liver. An elevated Alkaline phosphatase (ALP) was found in 18 (95%) of 19 patients,[9] and proteinuria was noted in 73% of the cases.[4,9,10] In our case, the ALP was within normal limits, and more suspicion was toward HCC; hence, serum AFP was done. A study consisting of 130 patients demonstrated that 5-year survival decreased from 72% to 43% when liver involvement of amyloid was noted.
The gold standard is the Congo red staining, which appears as apple-green birefringence under polarized light.[4,11,12]
AL amyloidosis involving the liver continues to have a poor prognosis, with survival of less than two years.[13] Furthermore, MM-associated amyloidosis, while rare, has an even worse prognosis.[5] Serum-free light chain levels, N-terminal pro-brain natriuretic peptide, and serum troponin T levels can be used as prognostic factors.[12] Levels of free light chain, rather than intact immunoglobulin levels, are better predictors of the outcome.[14] High-dose alkylating-based chemotherapy, in combination with hematopoietic stem cell transplantation for AL-amyloidosis, has been moderately effective, especially with limited cardiac involvement.[1,8]
CONCLUSION
Diagnosis of primary amyloidosis is difficult, and a high index of suspicion is required for timely diagnosis. Initially, a serum and urine immunofixation electrophoresis, especially the serum-free light chain proteins, can be ordered in suspected cases as a screening test. However, tissue biopsy is needed for diagnosing amyloidosis. Usually, the biopsy of the specified organ involved is the ideal and most sensitive. If unattainable due to risk factors or co-morbidities, an abdominal fat pad biopsy, which is approximately 80% sensitive, can be performed.
Acknowledgment
Dr. K. S. Nataraj - Department of Haematology. Dr. Sandeep Desai - Department of Radiology.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as the patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Multiple myeloma-a painful disease of the bone marrow. Semin Cell Dev Biol. 2021;112:49-58.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple myeloma-associated light chain amyloidosis involving heart, kidneys, and peripheral nerves: A case report. Med Case Rep Study Protoc. 2021;2:e0128.
- [CrossRef] [Google Scholar]
- Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid. 2014;21:221-4.
- [CrossRef] [PubMed] [Google Scholar]
- Kidney and liver involvement in monoclonal light chain disorders. Semin Nephrol. 2002;22:319-30.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic amyloidosis presenting as a large hepatic mass. Clin Gastroenterol Hepatol. 2012;10:e73-4.
- [CrossRef] [PubMed] [Google Scholar]
- Primary hepatic amyloidosis: Report of an unusual case presenting as a mass. Korean J Radiol. 2011;12:382-5.
- [CrossRef] [PubMed] [Google Scholar]
- Two cases with hepatic amyloidosis are suspected of having primary sclerosing cholangitis. Hepatol Res. 2013;43:911-6.
- [CrossRef] [PubMed] [Google Scholar]
- The liver in systemic amyloidosis: Insights from 123I serum amyloid P component scintigraphy in 484 patients. Gut. 1998;42:727-34.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic amyloidosis: Clinical appraisal in 77 patients. Hepatology. 1997;25:118-21.
- [CrossRef] [PubMed] [Google Scholar]
- Immunoglobulin light chain amyloidosis: 2013 update on diagnosis, prognosis, and treatment. Am J Hematol. 2013;88:416-25.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of N-terminal pro-brain natriuretic peptide and high-sensitivity troponin T levels in the natural history of transthyretin amyloid cardiomyopathy and their evolution after tafamidis treatment. J Clin Med. 2021;10:4868.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic failure due to myeloma-associated amyloidosis. J Gastroenterol. 1998;33:926-7.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in serum-free light chain rather than intact monoclonal immunoglobulin levels predicts outcome following therapy in primary amyloidosis. Am J Hematol. 2011;86:251-5.
- [CrossRef] [PubMed] [Google Scholar]






