Translate this page into:
Beyond anemia: Red cell distribution width as a universal biomarker in contemporary medicine
*Corresponding author: Rahul Garg, Department of Medicine, Farukh Hussain Medical College, Agra, Uttar Pradesh, India. gargrahul27@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Garg R. Beyond anemia: Red cell distribution width as a universal biomarker in contemporary medicine. J Hematol Allied Sci. doi: 10.25259/JHAS_12_2025
Abstract
Red cell distribution width (RDW), traditionally employed as a parameter for classifying anemia, has undergone a remarkable transformation in clinical medicine to emerge as a powerful prognostic biomarker across an extensive spectrum of pathological conditions. This comprehensive review meticulously examines RDW’s extraordinary evolution from its conventional role in hematology to its current standing as a versatile clinical indicator with broad applicability. A significant advantage of RDW is its automatic calculation by modern automated hematology analyzers as part of routine complete blood counts, rendering it exceptionally accessible and cost-effective as a biomarker in clinical settings worldwide. The complex pathophysiological mechanisms linking elevated RDW to various disease states involve sophisticated interactions between inflammatory cascades, oxidative stress pathways, nutritional deficiencies, bone marrow dysfunction, and metabolic dysregulations. The growing body of evidence supporting RDW’s clinical utility extends across multiple medical specialties, including cardiovascular medicine, hepatology, gastroenterology, endocrinology, pulmonology, infectious diseases, rheumatology, neurology, nephrology, and oncology. Despite its demonstrated broad applicability, significant challenges in standardization and interpretation persist, necessitating further research. Future investigative directions are focused on establishing disease-specific thresholds, integrating RDW into existing risk prediction models, and elucidating the molecular mechanisms that connect RDW elevation to pathological processes. As our understanding continues to deepen, RDW demonstrates exceptional promise for developing personalized approaches to disease monitoring and prognostication, firmly establishing its position as an invaluable tool in contemporary clinical practice.
Keywords
Biomarker
Cardiovascular disease
Hematological parameters
Mortality prediction
Prognostic indicator
Red cell distribution width
INTRODUCTION
Red cell distribution width (RDW) represents a fundamental hematological parameter that has emerged as a powerful prognostic biomarker across diverse medical conditions. Originally developed as a measure of variability in erythrocyte size, RDW has transcended its traditional role in anemia classification to become a valuable indicator of systemic disease processes. This transformation in our understanding of RDW’s clinical significance has occurred through extensive research over the past several decades.
RDW is automatically calculated by modern automated hematology analyzers as part of the complete blood count, making it one of the most readily available and cost-effective biomarkers in clinical practice. The parameter is typically expressed either as RDW-coefficient of variation (RDW-CV) or RDW-standard deviation (RDW-SD), with RDW-CV being the more commonly used format.[1] RDW-CV is calculated as the standard deviation of erythrocyte volumes divided by the mean corpuscular volume (MCV) and multiplied by 100 to express it as a percentage.[1,2] The normal reference range for RDWCV is typically between 11.5% and 14.5%, though this may vary slightly between laboratories. Modern automated hematology analyzers use flow cytometry principles to measure individual red cell volumes, enabling precise quantification of size variation.
This review aims to comprehensively examine the evolution of RDW from its conventional role in anemia classification to its current standing as a versatile prognostic biomarker, elucidate the pathophysiological mechanisms linking RDW elevation to various disease states, systematically evaluate the clinical utility of RDW across multiple medical specialties, identify current limitations in RDW implementation and propose future research directions, and assess the potential of RDW for developing personalized approaches to disease monitoring and prognostication.
This comprehensive review of RDW as a universal biomarker aligns with the focus on translational medicine and innovative diagnostic approaches, as it bridges fundamental hematological parameters with diverse clinical applications across multiple medical specialties.
MATERIAL AND METHODS
Literature search strategy
A comprehensive literature search was conducted using multiple electronic databases including PubMed/MEDLINE, Embase, Web of Science, Scopus, and the Cochrane Library. The following search terms were used in various combinations: “red cell distribution width,” “RDW,” “RDWCV,” “RDW-SD,” “biomarker,” “prognosis,” “mortality,” “risk prediction,” and “pathophysiology,” combined with specific disease terms for each medical specialty reviewed (e.g., “heart failure,” “cirrhosis,” “diabetes,” “COPD,” “sepsis,” and “cancer”).
Inclusion and exclusion criteria
Studies were included if they: (1) were written in English; (2) were original research articles, systematic reviews, or meta-analyses; (3) investigated RDW as a biomarker in any clinical context; and (4) were published between 2000 and 2024. Case reports with fewer than 10 subjects, conference abstracts, and letters to editors were excluded unless they presented novel findings not available elsewhere. Studies that did not report the methods used to measure RDW or that used non-standard measurement techniques were also excluded from the study.
Quality assessment
The methodological quality of included studies was assessed using the Newcastle-Ottawa Scale for observational studies, the Revised Cochrane risk-of-bias tool 2 for randomized trials, and AMSTAR-2 for systematic reviews and meta-analyses.
Data extraction and synthesis
Data were extracted using a standardized form that captured: Study design, population characteristics, RDW measurement methods, reference ranges, associated conditions, outcome measures, statistical methods, and main findings. Due to the heterogeneity of studies across different medical specialties, a narrative synthesis approach was adopted rather than a meta-analysis. Studies were grouped by medical specialty and condition to facilitate comparison of findings across similar contexts.
PATHOPHYSIOLOGICAL BASIS OF RDW AS A BIOMARKER
RDW serves as a prognostic indicator across diverse medical conditions through several interconnected biological mechanisms [Figure 1].
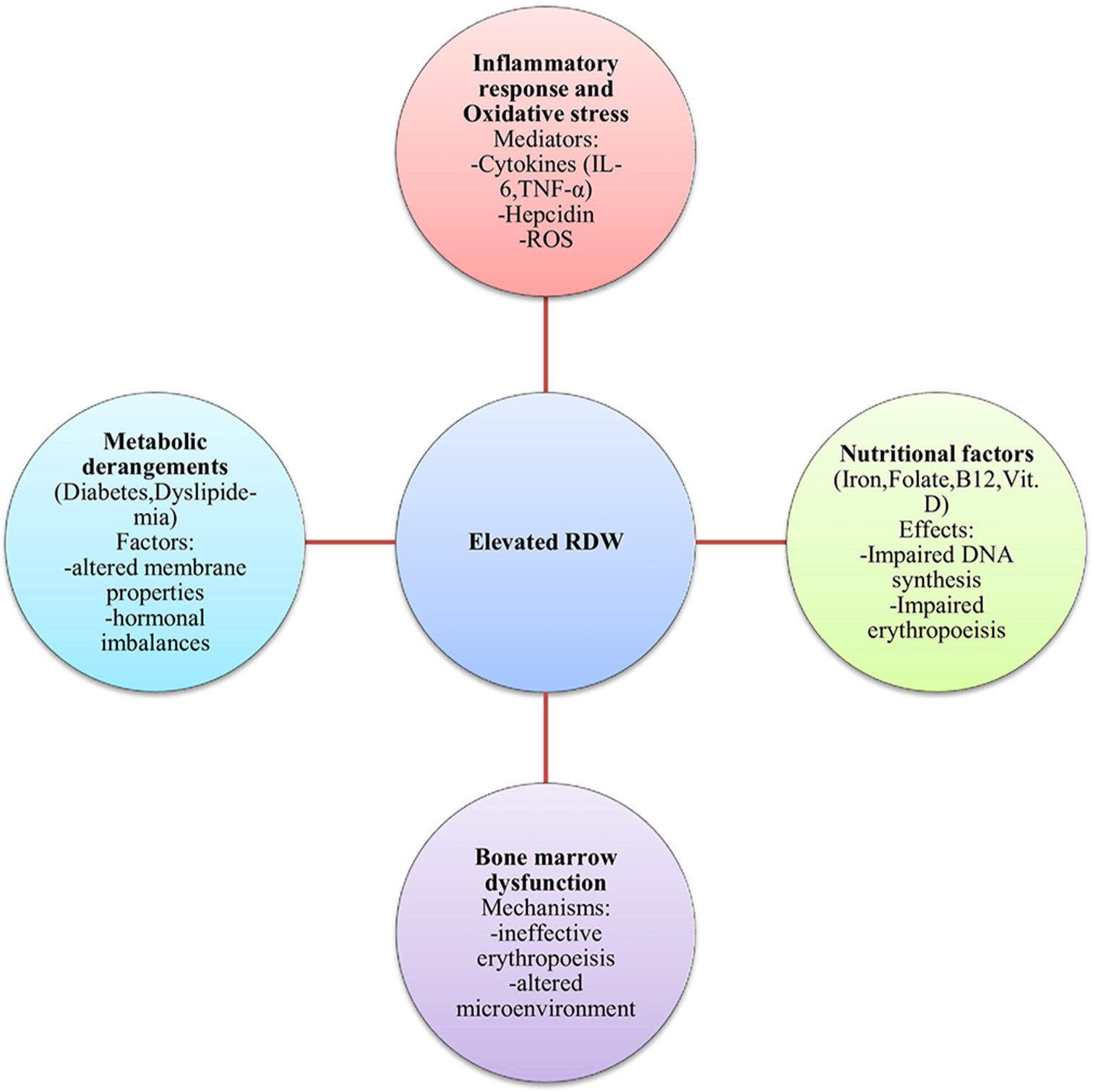
- Pathophysiological mechanisms contributing to elevated RDW. RDW: Red cell distribution width, IL-6: Interleukin-6, TNF-α: Tumor necrosis factor-alpha, ROS: Reactive oxygen species.
Inflammatory response and oxidative stress
Chronic inflammation significantly links elevated RDW to various diseases through multiple pathways. Pro-inflammatory cytokines (interleukin [IL]-6, tumor necrosis factor-alpha, and IL-1β) suppress erythroid progenitor cell development in bone marrow, leading to ineffective erythropoiesis and morphologically diverse red blood cells.[2]
Inflammation disrupts iron homeostasis through hepcidin upregulation, particularly in response to IL-6. Elevated hepcidin causes internalization and degradation of ferroportin, leading to functional iron sequestration within macrophages and enterocytes.[3] This functional iron deficiency results in iron-restricted erythropoiesis and increased red cell size heterogeneity.
Oxidative stress, often accompanying inflammation, directly impacts erythrocyte membrane integrity and lifespan. Reactive oxygen species cause lipid peroxidation, protein oxidation, and damage to cytoskeletal proteins, altering cell deformability and promoting premature erythrocyte removal. In response, the bone marrow increases production, releasing larger reticulocytes into circulation, thus increasing overall size variation.[4]
Nutritional factors
Iron deficiency is the most well-characterized nutritional cause of elevated RDW. During the transition from normal to iron-deficient erythropoiesis, the bone marrow produces a mixed population of normocytic and newly formed microcytic cells, significantly increasing erythrocyte size heterogeneity.[5]
Vitamin B12 and folate deficiencies affect RDW through their essential roles in DNA synthesis. Their deficiency leads to impaired DNA synthesis, affecting erythroid precursors and causing megaloblastic changes with ineffective erythropoiesis. The simultaneous presence of normal-sized mature erythrocytes and newly produced macrocytes creates a heterogeneous red cell population.[5]
Vitamin D deficiency links to increased RDW through direct and indirect mechanisms. Vitamin D receptors are expressed on erythroid precursor cells, and adequate Vitamin D is necessary for optimal erythropoiesis. In addition, Vitamin D deficiency associates with increased inflammatory cytokines and oxidative stress.[6]
Trace element deficiencies (copper, zinc, and selenium) can also contribute to RDW elevation as these elements serve as cofactors for enzymes involved in erythrocyte maturation and antioxidant defense.[7]
Bone marrow dysfunction
Primary bone marrow disorders (myelodysplastic syndromes, leukemias, and myeloproliferative neoplasms) directly affect erythropoiesis through genetic and epigenetic alterations in hematopoietic stem cells. These disorders feature ineffective erythropoiesis with abnormal proliferation and maturation, resulting in morphological abnormalities and elevated RDW.[8]
Bone marrow infiltration by malignant cells disrupts normal architecture and microenvironment, compromising the proliferation and differentiation of erythroid precursors. In multiple myeloma, RDW elevation correlates with disease severity and poorer outcomes.[8]
Iatrogenic factors, particularly chemotherapy and radiation, can induce bone marrow dysfunction through direct damage to hematopoietic stem cells, leading to decreased erythropoietic capacity and morphologically abnormal erythrocytes.[9]
Chronic systemic diseases (heart failure, chronic kidney disease, and inflammatory conditions) can cause secondary bone marrow dysfunction through inflammation, oxidative stress, uremic toxins, tissue hypoxia, and nutritional deficiencies.
Metabolic derangements
Glucose metabolism abnormalities influence RDW through multiple pathways. Chronic hyperglycemia promotes protein glycation, altering erythrocyte membrane properties and reducing cell deformability. Insulin resistance affects erythropoiesis through alterations in cellular metabolism and insulin signaling in erythroid progenitors.[10,11]
Lipid disorders impact RDW through effects on erythrocyte membrane composition. Dyslipidemia leads to excess cholesterol incorporation into erythrocyte membranes, altering membrane fluidity and function, affecting membrane-bound enzymes, and compromising erythrocyte deformability and survival.[12]
Hormonal imbalances (thyroid disorders and sex hormone fluctuations) significantly influence erythropoiesis and erythrocyte characteristics through effects on basal metabolic rate, iron metabolism, and erythropoietin production.[13,14]
Chronic kidney disease represents a complex metabolic disorder affecting erythropoiesis through decreased erythropoietin production, uremic toxin accumulation, chronic inflammation, and metabolic acidosis, all contributing to elevated RDW values that correlate with disease severity.[15]
CLINICAL APPLICATIONS
Various clinical conditions in which RDW serves as a marker are shown in Table 1.
| Hematological Iron deficiency anemia Megaloblastic anemia Hemolytic anemia Transfusion reactions Anemia of chronic disease Hereditary spherocytosis Sickle cell anemia Myelodysplastic syndrome |
Cardiovascular Heart failure Acute myocardial infarction Pulmonary hypertension Peripheral artery disease Hypertension Pulmonary embolism |
| Hepatological Hepatitis B, C, and E Chronic liver disease Non-alcoholic fatty liver Disease Autoimmune hepatitis Drug-induced liver injury |
Gastrointestinal Inflammatory bowel disease Autoimmune gastritis Celiac disease |
| Endocrinal Hashimoto thyroiditis Subclinical hypothyroidism Hyperthyroidism Hypothyroidism Diabetes mellitus |
Neurological Acute ischemic stroke Intracranial Hemorrhage Dementia Epilepsy |
| Respiratory Chronic obstructive pulmonary disease Community acquired pneumonia Tuberculosis |
Infectious Sepsis COVID-19 |
| Rheumatological Systemic lupus erythematosus Rheumatoid arthritis Ankylosing spondylitis |
Malignancies Lung cancer Gastric cancer Multiple myeloma Colorectal cancer Esophageal carcinoma |
| Renal Chronic Kidney Disease |
Hematological diseases
Since RDW is an index of anisocytosis, it has been used as an important marker in various hematological conditions.
Iron deficiency anemia
Megaloblastic anemia
Hemolytic anemia
Transfusion reactions
Anemia of chronic disease
Hereditary spherocytosis
Sickle cell anemia
Myelodysplastic syndrome.
RDW is normal in thalassemia.[17]
Cardiovascular diseases
RDW has demonstrated remarkable utility across various cardiovascular conditions:
Heart failure
The landmark CHARM Program and Duke Databank study established RDW as an independent predictor of mortality and morbidity in chronic heart failure and found that RDW had higher statistical association with outcome than routinely accepted measures of risk such as ejection fraction, NYHA functional class, and renal function.[19] Higher RDW correlates with increased mortality and hospitalization rate [Figures 2 and 3].
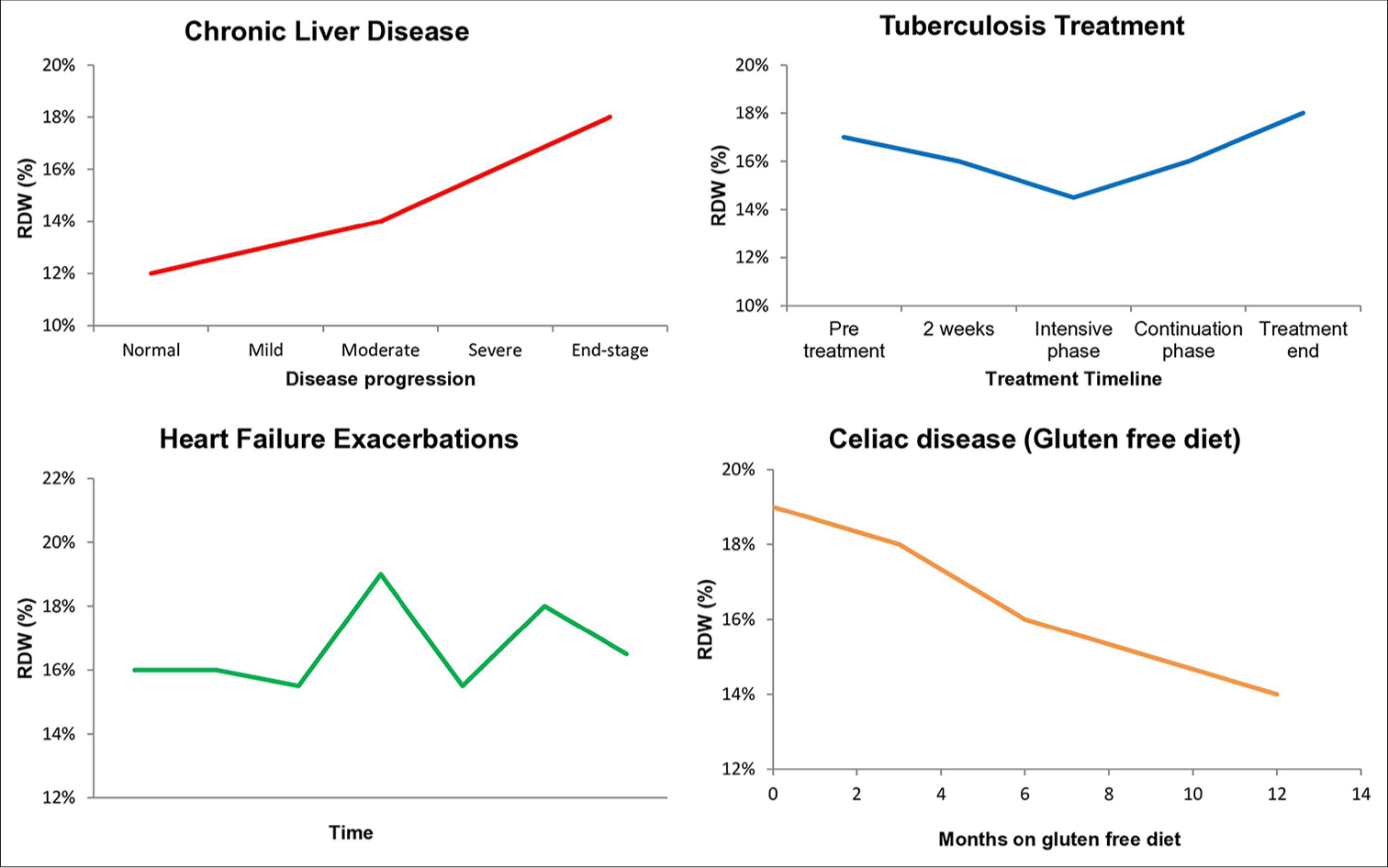
- RDW trends in various clinical conditions over time. RDW: Red cell distribution width.
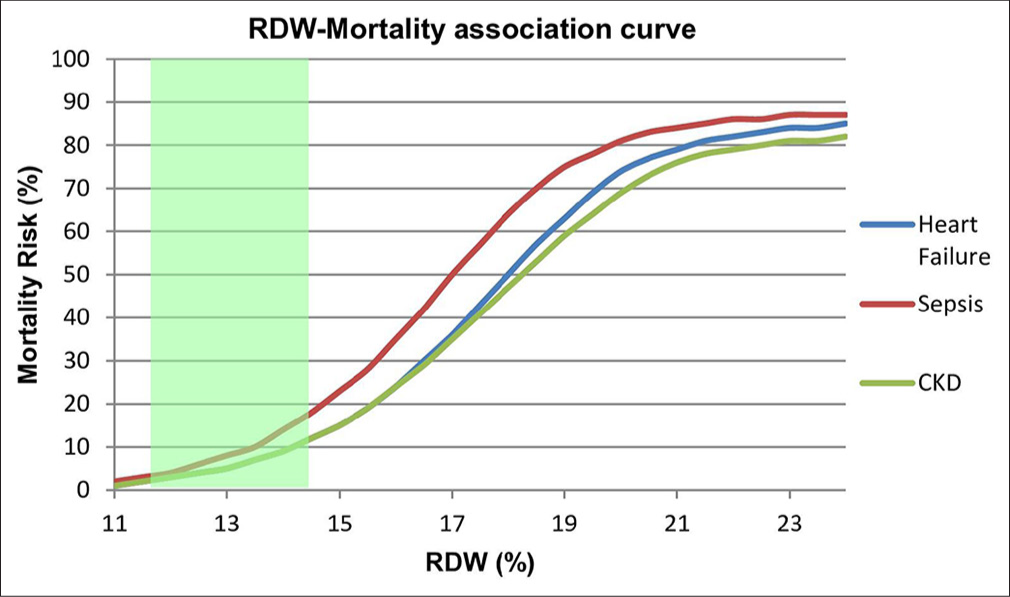
- Association between RDW values and mortality risk across different conditions. Normal RDW range (11.5–14.5%) is indicated by green shading. Note the steeper curve for sepsis, indicating higher mortality risk at lower RDW values compared to heart failure and CKD. Values above 21% are associated with mortality rates exceeding 70% across all conditions. RDW: Red cell distribution width, CKD: Chronic kidney disease
Acute myocardial infarction
In acute coronary syndromes, higher RDW values correlate significantly with increased mortality post-myocardial infarction.[20] A study established a graded independent relation between higher levels of RDW and the risk of death and cardiovascular events in people with prior myocardial infarction but no symptomatic heart failure at baseline.[20]
Pulmonary hypertension
Elevated RDW correlates with disease severity and worse outcomes. RDW is independently associated with death in patients with Pulmonary Hypertension and performs better as a prognostic indicator than N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).[21]
Peripheral arterial disease
RDW has been found to be associated with high risk of mortality in patients with peripheral artery disease (PAD).[22] There was a 10% increase in mortality risk with every 1% increment in RDW in patients with PAD.[22] Higher RDWto-albumin ratio (RAR) indicates increased disease risk, particularly in diabetic patients.[23]
Hypertension
Elevated RDW correlates with higher blood pressure values and target organ damage. RDW elevation has been found in pre-hypertension and hypertension, and associated with increased risk of mortality and cardiovascular events in people with hypertension.[16]
Pulmonary embolism
High RDW has been found to be associated with worse hemodynamic parameters and seems to aid in the risk stratification of patients with acute pulmonary embolism.[24] High level of RDW has also been shown to be an independent predictor of chronic thromboembolic pulmonary hypertension in patients with pulmonary embolism.[25]
Liver diseases
RDW has emerged as a significant marker in hepatic disorders:
Hepatitis B, C, and E
RDW levels have been found to be significantly increased in patients with hepatitis B, C, and E and associated with its severity. Moreover, RDW values are an independent predicting factor for the 3-month mortality rate in patients with hepatitis B.[26,27] Recent research indicates that RDW-SD shows better correlation with fibrosis stage than RDW-CV in hepatitis B.[1]
Chronic liver disease
Studies demonstrate a progressive increase in RDW with disease severity [Figure 2]. It can be combined with other traditional markers for predictive and prognostic purposes in chronic liver disease.[26] High RDW values have shown a correlation with the severity of cirrhosis in terms of Child-Pugh scores and model for end-stage liver disease (MELD) scores.[27]
Non-alcoholic fatty liver disease (NAFLD) and hepatic fibrosis
Studies have demonstrated a possible link between RDW and the degree of fibrosis in NAFLD.[26]
Autoimmune hepatitis (AIH)
RDW has shown usefulness as a non-invasive marker to predict and diagnose AIH along with other markers and could be used as a possible prognostic marker. A significant positive correlation has been noted between the degree of inflammation and RDW and immunoglobulin G.[26]
Drug-induced liver injury
Herbal drugs can cause severe liver injury and higher RDW, which correlates with worse outcomes.[28]
Gastrointestinal diseases
RDW has been linked to some gastrointestinal conditions:
Inflammatory bowel disease
RDW can be used as a sensitive and specific marker for detecting ulcerative colitis and Crohn’s disease.[18,27]
Autoimmune gastritis
RDW values have been found to be significantly increased in patients with autoimmune gastritis.[27]
Celiac disease
Increased RDW has been found to be the most frequent hematological abnormality in these patients, followed by anemia and iron deficiency.[27] Elevated RDW values have been shown to be a predictor of intestinal atrophy in these patients. Many studies have demonstrated improvement in RDW values with gluten free diet [Figure 2].[27]
Endocrine disorders
The role of RDW in endocrine conditions has been extensively studied:
Thyroid function
Some studies have indicated that elevated RDW level is significantly associated with Hashimoto thyroiditis, subclinical hypothyroidism, hyperthyroidism, and hypothyroidism.[29]
Diabetes mellitus and insulin resistance
Low RDW is associated with increased incidence of diabetes mellitus and could be used as a surrogate marker for lower HbA1c.[11] Higher RDW values are associated with increased chances of developing cardiovascular disease and nephropathy in diabetics. A study demonstrated significant association of RDW values with homeostatic model assessment (HOMA) 2%B and HbA1c and suggested that RDW may be more important in increasing insulin secretion than in insulin resistance. HOMA2%B is an index of basic insulin secretion, which represents the ability of islet β-cells to compensate against insulin resistance.[10] High RAR is associated with an increased risk of developing retinopathy.[23]
Respiratory diseases
RDW has demonstrated significant prognostic value in respiratory conditions:
Chronic obstructive pulmonary disease (COPD)
Higher RDW is associated with increased mortality risk. RDW levels may be used as a marker of systemic inflammation in COPD. It could also be used as a predictor of future COPD outcome.[30]
Community-acquired pneumonia (CAP)
A study indicated that the higher RDW value is associated with short-term adverse outcomes in CAP patients. Combination of RDW, pneumonia severity index (PSI), and CURB-65 proved to be best in predicting CAP 90-day mortality risk.[31]
Tuberculosis (TB)
RDW changes during anti-TB treatment can indicate treatment response [Figure 2]. A study showed that RDW of treatment naïve TB patients was lower than corresponding RDW of intensive phase treatment completed patients.[32]
Infectious diseases
RDW’s utility in infectious conditions has gained increasing recognition:
Sepsis
RDW values >15.8% predict higher mortality in patients with severe sepsis and septic shock [Figure 3].[33]
COVID-19
Elevated RDW is associated with increased mortality risk. A study demonstrated that patients whose RDW increased during hospitalization had higher mortality compared with those whose RDW did not change.[34]
Connective tissue diseases
RDW has shown significant utility in monitoring connective tissue diseases:
Systemic lupus erythematosus (SLE)
A study indicated that RDW correlates strongly with disease activity score SLE Disease Activity Index 2000 (SLEDAI-2K) along with serum immunoglobulin M, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), and glucocorticoid treatment decreased both SLEDAI-2K and RDW.[35]
Rheumatoid arthritis, ankylosing spondylitis
Higher RDW associates with increased disease activity. Multiple studies have reported the association between increased RDW values and rheumatoid arthritis and ankylosing spondylitis independent of anemia and nutritional deficiencies.[36]
Neurological disorders
RDW has demonstrated important prognostic value in neurological conditions:
Acute ischemic stroke
Few studies have suggested that higher RDW correlates with increased stroke severity and serves as a predictor of poor outcomes.[37]
Intracranial hemorrhage (ICH)
A study has proven RDW to be a robust and independent predictor of 30-day mortality in non-traumatic ICH patients. Higher RDW values were associated with increased neurologic impairment severity as measured by GCS and with increasing ICH scores.[38]
Dementia
Strong association between higher RDW values and dementia in non-anemic patients have been shown by a study.[39]
Epilepsy
A study demonstrated that RDW was an independent predictor of in-hospital mortality in patients with seizures admitted to intensive care unit (ICU), and those with higher RDW had a longer stay in the ICU.[40]
Renal diseases
The relationship between RDW and kidney function has been well-documented:
Chronic kidney disease
RDW shows progressive increases with declining kidney function as manifested by worsening glomerular filtration rate, serving as a potential marker for disease progression [Figure 3].[15] A significant positive correlation was noted between RDW and early kidney damage, as indicated by microalbuminuria.
Malignancies
RDW has emerged as a valuable prognostic marker in various cancers:
Lung cancer
Higher RDW values correlate with advanced disease stage and poor prognosis.[41]
Gastric cancer
Studies have demonstrated higher RDW values in gastric cancer and are associated with worse survival outcomes.[27]
Multiple myeloma
Increased RDW levels predict poorer outcomes in symptomatic multiple myeloma patients.[8]
Colorectal cancer
Studies have shown RDW’s potential as a diagnostic and prognostic marker in colorectal cancer patients.[27]
Esophageal carcinoma
Increased RDW was then found to be an independent prognostic factor for cancer specific survival, with mortality being nearly twice higher in patients with higher RDW.[27]
CLINICAL IMPLEMENTATION AND FUTURE DIRECTIONS
Current limitations and challenges
The clinical implementation of RDW as a biomarker faces several important limitations that must be addressed for optimal utilization. As a non-specific marker, elevated RDW can result from multiple conditions, making interpretation challenging in complex cases. Various clinical and demographic factors can act as confounders, potentially masking or exaggerating RDW’s true significance in certain populations. Standardization issues persist, with different analytical methods producing varying results across laboratories and healthcare systems. In addition, the time-dependent variations in RDW values necessitate careful consideration of measurement timing in clinical decision-making.
Implementation strategies
Despite these limitations, several approaches can enhance RDW’s clinical utility:
Contextual Interpretation: RDW values should be interpreted within the specific clinical context rather than in isolation, considering patient demographics, comorbidities, and concurrent laboratory findings
Serial Monitoring: Tracking RDW changes over time within individual patients often provides more valuable information than single measurements, particularly for assessing treatment response or disease progression
-
Multiparameter Approaches: Combining RDW with complementary biomarkers can enhance diagnostic and prognostic accuracy. For example:
In cardiovascular risk assessment: RDW + NTproBNP + high-sensitivity troponin
In liver disease: RDW + MELD score components
In inflammatory conditions: RDW + CRP + ESR
Risk Stratification Tools: Incorporating RDW into specialty-specific risk calculators to identify high-risk patients requiring more intensive monitoring or intervention.
Future research priorities
Future research priorities should focus on standardizing RDW measurement and reporting across different platforms to ensure consistency and comparability of results. The development of disease-specific RDW thresholds for various clinical applications would significantly enhance its diagnostic and prognostic utility. Integration of RDW into existing risk prediction models represents another promising avenue, potentially improving risk stratification across multiple conditions. Investigation of RDW’s role in emerging medical conditions and understanding the molecular mechanisms linking RDW to various disease states will be crucial for expanding its clinical applications.
Integration into existing risk prediction models
A particularly promising avenue for future research is the integration of RDW into established risk prediction tools. Current prognostic models in various medical specialties could benefit from incorporating RDW, potentially enhancing their predictive accuracy. Several preliminary studies have already demonstrated improved prognostic performance when RDW is added to existing models:
In cardiovascular medicine, adding RDW to the GRACE score improved risk prediction for adverse outcomes after acute coronary syndromes[42]
In hepatology, incorporating RDW into MELD and Child-Pugh scores enhanced their ability to predict mortality in patients with cirrhosis[26]
In pneumonia, combining RDW with CURB-65 and PSI scores increased predictive accuracy for 90-day mortality[31]
These preliminary findings suggest that RDW could serve as a valuable addition to existing risk stratification tools, potentially improving clinical decision-making across various medical disciplines.
Figure 4 represents a comprehensive overview of RDW as a universal biomarker in contemporary medicine. This integrated visualization illustrates how RDW has evolved from a simple hematological parameter to a versatile prognostic biomarker with applications across multiple medical disciplines.
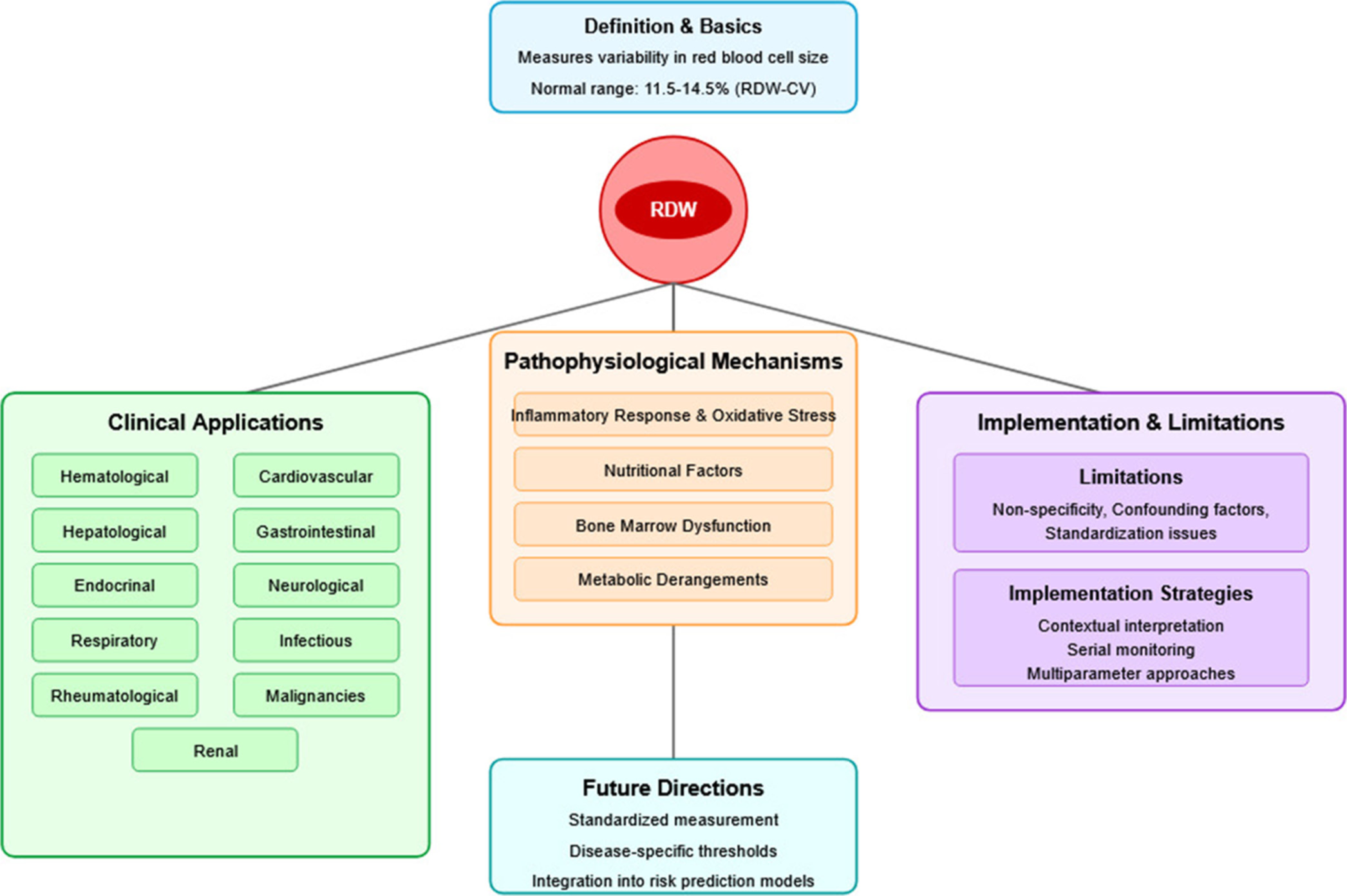
- Comprehensive overview of red cell distribution width as a universal biomarker in contemporary medicine.
CONCLUSION
The evolution of RDW from a simple anemia marker to a powerful prognostic indicator across numerous medical specialties represents a remarkable advancement in clinical biomarkers. Its widespread availability, cost-effectiveness, and substantial predictive value make it an invaluable tool in modern medicine. The extensive evidence supporting RDW’s utility in cardiovascular diseases, hepatic disorders, endocrine conditions, respiratory diseases, and more recently in neurological disorders, renal diseases, and malignancies demonstrates its exceptional versatility. Despite existing limitations, ongoing research continues to expand our understanding of the mechanisms linking RDW to pathological states, potentially leading to more personalized approaches in disease monitoring and prognostication. As standardization improves and disease-specific thresholds are established, RDW’s role in clinical practice will likely become even more significant.
Ethical approval:
The Institutional Review Board approval is not required.
Declaration of patient consent:
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The author confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- RDW-SD is superior to RDW-CV in reflecting liver fibrosis stage in patients with chronic hepatitis B. Infect Drug Resist. 2023;16:6881-91.
- [CrossRef] [PubMed] [Google Scholar]
- Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628-32.
- [CrossRef] [PubMed] [Google Scholar]
- Liver iron sensing and body iron homeostasis. Blood. 2019;133:18-29.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10:1923-40.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormal erythropoiesis and the pathophysiology of chronic anemia. Blood Rev. 2014;28:49-66.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D status and elevated red cell distribution width in community-dwelling adults: Results from the National Health and Nutrition Examination Survey 2001-2006. J Nutr Health Aging. 2017;21:1176-82.
- [CrossRef] [PubMed] [Google Scholar]
- Trace element status (Iron, zinc, copper, chromium, cobalt, and nickel) in iron-deficiency anaemia of children under 3 years. Anemia. 2014;2014:718089.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated red blood cell distribution width as a simple prognostic factor in patients with symptomatic multiple myeloma. Biomed Res Int. 2014;2014:145619.
- [CrossRef] [PubMed] [Google Scholar]
- Red cell differential width (RDW) as a predictor of survival outcomes with palliative and adjuvant chemotherapy for metastatic penile cancer. Int Urol Nephrol. 2020;52:2301-6.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship between red blood cell distribution and islet β-cell function indexes in patients with type 2 diabetes. BMC Endocr Disord. 2021;21:7.
- [CrossRef] [PubMed] [Google Scholar]
- Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. J Intern Med. 2014;276:174-83.
- [CrossRef] [PubMed] [Google Scholar]
- Red blood cell distribution width is associated with carotid atherosclerosis in people with type 2 diabetes. J Diabetes Res. 2018;2018:1792760.
- [CrossRef] [PubMed] [Google Scholar]
- Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166:1380-8.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand J Clin Lab Invest. 2008;68:745-8.
- [CrossRef] [PubMed] [Google Scholar]
- Importance of mean red cell distribution width in hypertensive patients. Cureus. 2016;8:e902.
- [CrossRef] [Google Scholar]
- Haematological, biochemical and inflammatory parameters in inactive Behçet's disease. Its association with red blood cell distribution width. Clin Hemorheol Microcirc. 2014;56:319-24.
- [CrossRef] [PubMed] [Google Scholar]
- Red cell distribution width as a marker of activity in inflammatory bowel disease: A narrative review. Ann Gastroenterol. 2020;33:348-54.
- [CrossRef] [PubMed] [Google Scholar]
- Red cell distribution width as a novel prognostic marker in heart failure: Data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50:40-7.
- [CrossRef] [PubMed] [Google Scholar]
- Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163-8.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol. 2009;104:868-72.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol. 2011;107:1241-5.
- [CrossRef] [PubMed] [Google Scholar]
- Association between red cell distribution width-and-albumin ratio and the risk of peripheral artery disease in patients with diabetes. Front Endocrinol (Lausanne). 2024;15:1272573.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of admission red cell distribution width as a predictor of early mortality in patients with acute pulmonary embolism. Am J Cardiol. 2012;109:128-34.
- [CrossRef] [PubMed] [Google Scholar]
- Red cell distribution width: A new predictor for chronic thromboembolic pulmonary hypertension after pulmonary embolism. Chron Respir Dis. 2014;11:73-81.
- [CrossRef] [PubMed] [Google Scholar]
- The role of red cell distribution width as a prognostic marker in chronic liver disease: A literature review. Int J Mol Sci. 2023;24:3487.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol. 2017;23:4879-91.
- [CrossRef] [PubMed] [Google Scholar]
- Increased red cell distribution width predicts severity of drug-induced liver injury: A retrospective study. Sci Rep. 2021;11:773.
- [CrossRef] [PubMed] [Google Scholar]
- Association between red blood cell distribution width and thyroid function. Front Endocrinol (Lausanne). 2022;12:807482.
- [CrossRef] [PubMed] [Google Scholar]
- Red blood cell distribution and survival in patients with chronic obstructive pulmonary disease. COPD. 2013;10:416-24.
- [CrossRef] [PubMed] [Google Scholar]
- The role of red blood cell distribution width in the severity and prognosis of community-acquired pneumonia. Can Respir J. 2021;2021:8024024.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of anti-tuberculosis drugs on hematological profiles of tuberculosis patients attending at University of Gondar Hospital, Northwest Ethiopia. BMC Hematol. 2016;16:1.
- [CrossRef] [PubMed] [Google Scholar]
- Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am J Emerg Med. 2013;31:545-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 infection. JAMA Netw Open. 2020;3:e2022058.
- [CrossRef] [PubMed] [Google Scholar]
- Red blood cell distribution width is a potential index to assess the disease activity of systemic lupus erythematosus. Clin Chim Acta. 2013;425:202-5.
- [CrossRef] [PubMed] [Google Scholar]
- Red cell distribution width in rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:38-42.
- [CrossRef] [PubMed] [Google Scholar]
- Red cell distribution width (RDW) index as a predictor of severity of acute Ischemic stroke: A correlation study. Adv J Emerg Med. 2019;4:e24.
- [Google Scholar]
- Red cell distribution width is associated with 30-day mortality in patients with spontaneous intracerebral hemorrhage. Neurocrit Care. 2021;34:825-32.
- [CrossRef] [PubMed] [Google Scholar]
- The red cell distribution width and anemia in association with prevalent dementia. Alzheimer Dis Assoc Disord. 2014;28:99-105.
- [CrossRef] [PubMed] [Google Scholar]
- Red blood cell distribution width predicts in-hospital mortality in patients with a primary diagnosis of seizures in the ICU: A retrospective database study. Neurol Sci. 2022;43:499-506.
- [CrossRef] [PubMed] [Google Scholar]
- Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One. 2013;8:e80240.
- [CrossRef] [PubMed] [Google Scholar]
- Combined value of red blood cell distribution width and global registry of acute coronary events risk score on predicting long-term major adverse cardiac events in STEMI patients undergoing primary PCI. Oncotarget. 2018;9:13971-80.
- [CrossRef] [PubMed] [Google Scholar]






