Translate this page into:
A case where the histiocytes helped thread those crystalline needles
*Corresponding author: Kriti Chauhan, Department of Pathology, Metropolis Pvt. Ltd, Chandigarh, India. kritichauhan25@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chauhan K, Singh G. A case where the histiocytes helped threads those crystalline needles. J Hematol Allied Sci. 2023;3:67-70. doi: 10.25259/JHAS_21_2023
Abstract
Plasma cell neoplasms involve excessive immunoglobulin (Ig) secretion which is the main culprit. Hence, they are the most sought after cells in bone marrow whenever plasma cell dyscrasias is suspected. In their absence, the diagnosis becomes questionable. However, if one can identify those Igs disguising themselves as crystals in histiocytes, the path becomes clearer. We present one such case of pancytopenia where an incidental finding of these crystal storing histiocytes helped clinch the diagnosis of a monoclonal gammopathy which was otherwise suspected to be a consequence of anti-tuberculous therapy.
Keywords
Crystal storing histiocytes
Plasma cells
Monoclonal
Immunoglobulins
INTRODUCTION
Crystal storing histiocytosis (CSH) is a disorder of faulty intralysosomal degradation of immunoglobulins (Igs) by histiocytes which results in the accumulation of partially lysed structures forming crystals in the cytoplasm.[1] This improper enzymatic degradation of Ig is sequelae of acquired resistance due to structural alterations in the amino acid sequence associated with a monoclonal or polyclonal proliferation of plasma cells. Other than the Igs, some foreign bodies can also crystallize in the macrophages.[2] CSH may be localized (confined to a single organ) or generalized (multiple organs involved) depending on the etiology. Figure 1 shows a flow chart describing the different types of CSH and their probable behavior.

- Flow chart showing the types of crystal storing histiocytosis.
CASE REPORT
A 62-year-old female patient with a history of pulmonary tuberculosis presented with extreme generalized weakness and chest pain. She had completed her anti-tubercular (anti-TB) therapy a few months back and confirmed admissions in several hospitals for blood transfusions in view of anemia. On investigating further, the hemogram revealed pancytopenia (Hb = 8.4 g/dL, red blood cell count = 2.90 × 10^6/µL, total leukocyte count = 1.4 × 10^3/µL, and platelet count = 36 × 10^3/µL) which was initially suspected to be anti-TB therapy induced. However, the counts failed to recover despite the discontinuation of therapy. Hence, a review of peripheral smear (PS) along with bone marrow examination was planned to rule out the possibility of a coexisting hematological disorder. PS revealed normocytic normochromic anemia with rouleaux formation. White blood cells were moderately reduced in number and showed normal distribution. Platelets were markedly reduced on the smear. An occasional giant platelet was seen.
The corrected reticulocyte count was 3.45% suggesting an appropriate bone marrow regenerative response. Bone marrow aspirate yielded a dry tap. Bone marrow biopsy was hypercellular and showed a prominent population of mast cells diffusely present throughout and having abundant cytoplasm containing eosinophilic granules. No clustering or spindle cell forms were noted. Along with this, there were interstitially arranged plasma cells revealing intracytoplasmic inclusions focally [Figure 2]. A noteworthy finding was the focal presence of histiocytic cells bearing intracytoplasmic needle like eosinophilic crystalline inclusions [Figure 3a]. Trilineage hematopoiesis was suppressed. No fibrosis was noted. Based on these observations, it was postulated that the bone marrow was recovering from the suppression due to anti-tuberculous therapy. The mastocytic prominence was a reactive phenomenon of recovering marrow. In addition, the crystal storing histiocytes in a background of scanty yet prominent plasma cells along with rouleaux formation on PS drew our attention and warranted evaluation for plasma cell dyscrasias. Serum and urine protein electrophoresis revealed a monoclonal band having a concentration of 2.66 g/dL in serum [Figure 3b]. Hence, a diagnosis of Monoclonal Gammopathy of Undetermined Significance was offered since the clonal population of plasma cells was <10%, the “M” protein concentration was <3 g/dL, and anemia/bone lesions though present were not attributable to plasma cell disorder. The patient is being treated and closely followed up to prevent conversion to multiple myeloma.
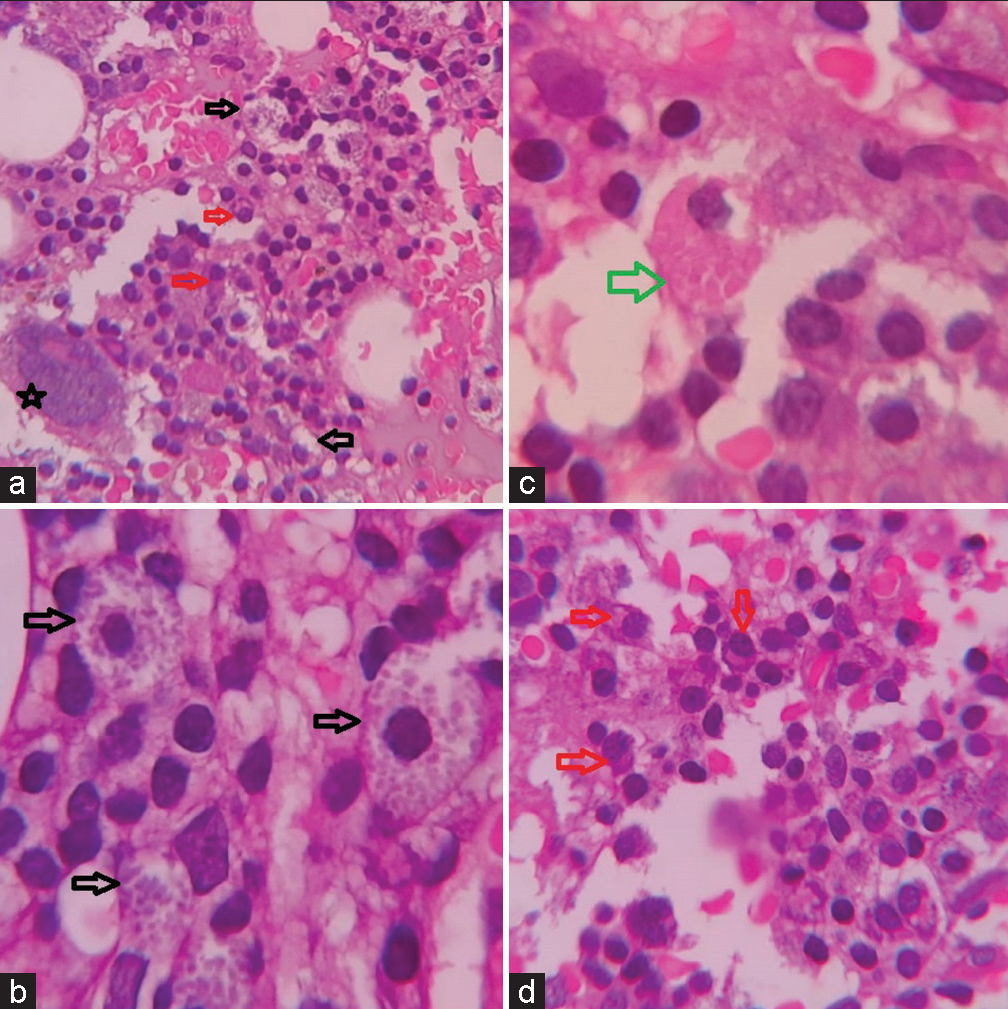
- (a) Low power view of bone marrow biopsy (×100 H&E), (b-d) high power view of bone marrow biopsy showing mast cells (black arrow), plasma cells (red arrow), and crystal storing histiocytes (green arrow) (×400 H&E).
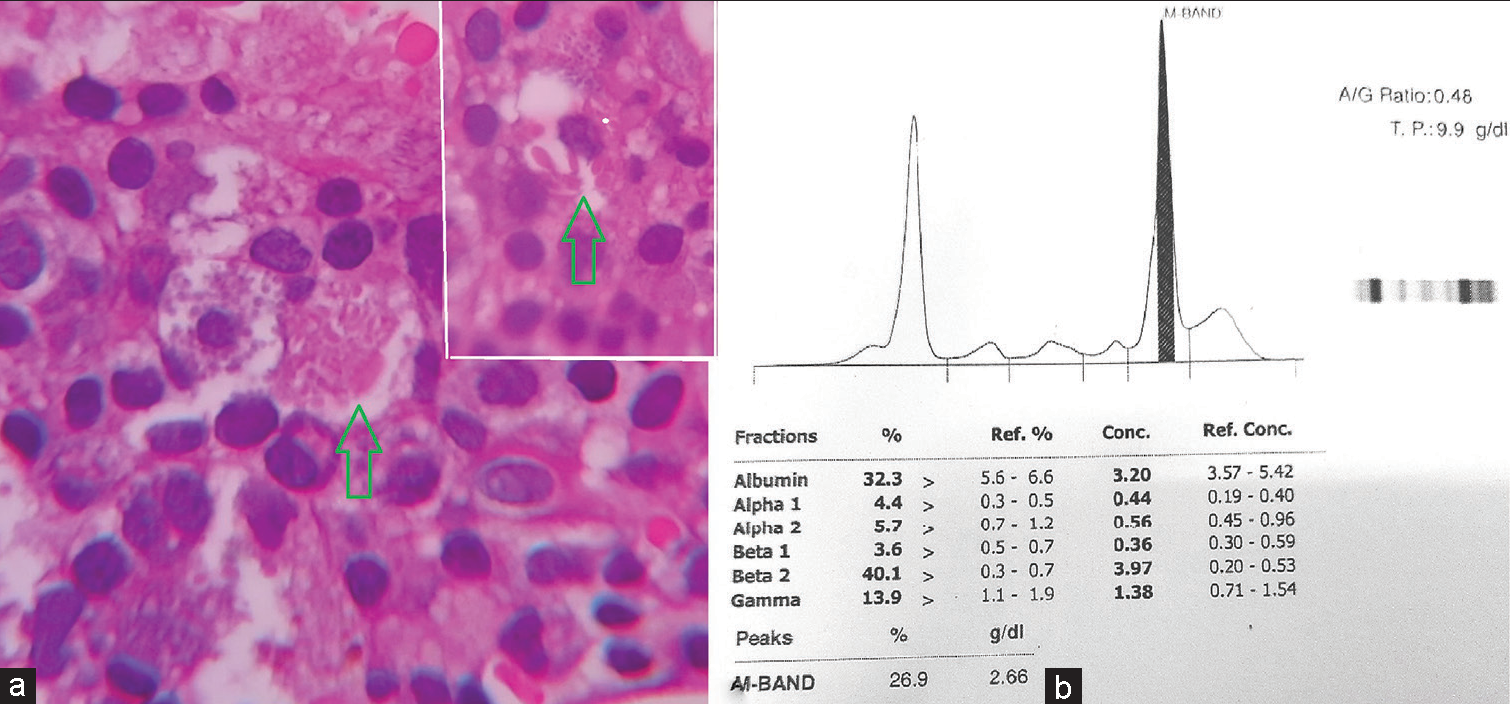
- (a) High power view of a histiocyte showing needle shaped crystals (green arrow). Inset showing another similar histiocyte (×400 H&E). (b) Graph of serum protein electrophoresis depicting the monoclonal band.
DISCUSSION
CSH is not a disease per se but a process hinting toward excessive production or improper degradation of Igs. Approximately, 90% of the cases of CSH are associated with monoclonal plasma cell disorders with a predilection toward kappa chain overproduction and crystallization.[1,3,4] Intracytoplasmic eosinophilic inclusions in macrophages can also be seen in many other conditions such as malakoplakia, rosai-dorfman disease, Whipple’s disease, and Gaucher’s disease but the composition, shape, and color of the inclusions along with the background cell population are different.[1,5] Malakoplakia is an inflammatory condition associated with improper phagocytosis of bacterial products. The macrophages show characteristic targetoid inclusions called “MichaelisGutmann” bodies which represent remnants of phagosomes mineralized by iron and calcium deposits. Rosai-Dorfman disease is a histiocytic disorder involving skin and lymph nodes, characterized by cytoplasmic ingestion of intact inflammatory cells, called emperipolesis. Whipple disease is a multisystem infection caused by the bacterium tropheryma whipplei which colonies in the cytoplasm of macrophages in the small intestinal mucosa, highlighted by periodic acid schiff stain. Finally, Gaucher disease is a lysosomal storage disorder characterized by macrophagic accumulation of glucocerebrosides imparting a crinkled paper eosinophilic appearance to the cytoplasm.[5] The correlation of clinical findings with the morphological analysis can help reach the correct diagnosis. Finding these few scattered crystals containing histiocytes despite a sparse plasma cell infiltrate helped us clinch the diagnosis. Hence, we as pathologists have a significant role to play in patient care and cannot afford to miss even the minutest details.
CONCLUSION
We share this case to bring to light the importance of careful PS and bone marrow examination in such cases where the clinical scenario suspected reactivation or spread of an existing disease (tuberculosis). Having excluded the same, an effort should be made to find the actual cause and rule out the possibility of some other coexisting pathology that is preventing the desired response. The presence of a few crystal storing histiocytes and plasma cells was significant enough to catch our attention and carry out further investigations which led to the diagnosis of a malignant disease in its incipient stage where timely intervention can contain the development of a full blown disease.
Acknowledgment
The authors are extremely thankful to the technical staff for their help and support
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Crystal-storing histiocytosis: The iceberg of more serious conditions. Diagnostics (Basel). 2023;13:271.
- [CrossRef] [PubMed] [Google Scholar]
- Crystal storing histiocytosis: Unusual clinical presentations in two patients. Ann Diagn Pathol. 2019;40:13-7.
- [CrossRef] [PubMed] [Google Scholar]
- Crystal-storing histiocytosis: A clinicopathological study of 13 cases. Histopathology. 2016;68:482-91.
- [CrossRef] [PubMed] [Google Scholar]
- Crystal-storing histiocytosis in bone marrow: A clinicopathologic study of eight cases and review of the literature. Am J Clin Pathol. 2018;149:148-63.
- [CrossRef] [PubMed] [Google Scholar]
- Crystal-storing histiocytosis: Report of a rare case presenting with pathological fracture of femur. Is there more to the entity? Int J Surg Pathol 2017. ;. ;25:458-61.
- [CrossRef] [PubMed] [Google Scholar]






