Translate this page into:
A retrospective analysis of response rates and predictors of response to rituximab in management of primary immune thrombocytopenia: Second line and beyond
*Corresponding author: Shailendra Prasad Verma, Department of Clinical Hematology, King George’s Medical University, Lucknow, Uttar Pradesh, India. spverma1998@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Verma SP, Pavecha P, Tripathi AK, Singh BK, Shukla A, Verma DP, et al. A retrospective analysis of response rates and predictors of response to rituximab in management of primary immune thrombocytopenia: Second line and beyond. J Hematol Allied Sci. 2024;4:59-66. doi: 10.25259/JHAS_29_2024
Abstract
Objectives:
Rituximab is an important second-line option for the treatment of chronic/persistent primary immune thrombocytopenia (ITP). This study aimed to analyze the overall response rates (ORRs) and various factors affecting progression-free survival with rituximab treatment. This can help choose this drug as a second-line option for the best-suited candidates.
Material and Methods:
We retrospectively analyzed our departmental data of 25 ITP patients treated with rituximab between 2012 and 2020. All patients received rituximab post-first line. Patients with chronic or persistent ITP receiving at least 4 weekly doses of rituximab were included in the study. Patients receiving both low-dose and standard-dose rituximab were included in the study.
Results:
The median age of patients was 25 years, with a male-female ratio of 1:2. Most patients received rituximab as third-line (56%) or fourth-line (36%) treatment option. Overall, the long-term response rate at a median follow-up of 47.6 months was 60%, while the early response rate at 4 weeks, 8 weeks, and 6 months from the first dose of rituximab was 80%, 60%, and 60%, respectively. Forty per cent of patients could maintain a platelet count >50 × 109/L at 6 months and in the long term. Response at 2 weeks, 4 weeks, early use of rituximab (<12 months), and age >20 years were associated with significantly better progression-free survival.
Conclusion:
Rituximab is still a very promising option for primary ITP. Short and definite duration of treatment, good safety profile, and impressive ORRs make this agent a viable option for ITP patients in the current era. Response at 2 weeks, 4 weeks, early use of rituximab (<12 months), and age >20 years were associated with significantly better progression-free survival.
Keywords
Immune thrombocytopenia
Rituximab
Relapsed/refractory immune thrombocytopenia
Response rate
Progression-free survival
INTRODUCTION
Thrombocytopenia is a common hematological abnormality defined by a platelet count of <150 × 109/L and needs evaluation once it is <100 × 109/L. Causes of thrombocytopenia are increased destruction, reduced production, or peripheral pooling of platelets. Immune thrombocytopenia (ITP), previously known as ITP purpura, is a diagnosis of exclusion, and this hematological disorder is characterized by immune-mediated increased destruction along with decreased production of platelets.[1] ITP can be primary (idiopathic) or secondary (due to human immunodeficiency virus [HIV], hepatitis C virus [HCV], or autoimmune disorders). Based on the duration of symptoms, it can be newly diagnosed or recent onset (<3 months), persistent (3–12 months), or chronic (>12 months). Pediatric ITP usually recovers in up to 80% of cases; contrary to this, adult ITP usually has a chronic relapsing-remitting course.[1]
Steroids, intravenous immunoglobulins, and anti-RhD are the first-line treatment options for primary ITP, while secondary ITP needs the treatment of the underlying cause. Treatment indications are based on symptoms rather than platelet count, but it is prudent to start treatment once platelet count is below <30 × 109/L because platelet count below this threshold is associated with increased bleeding. Only 60–70% of patients respond to initial steroid treatment, and most of them relapse during steroid tapering. Steroids cannot be given for more than 4–6 weeks due to associated side effects.[1]
Trombopoietinmimetics (TPO-mimetics), eltrombopag and romiplostim, rituximab, and splenectomy are feasible options in the second line.[2] Splenectomy is the most effective option but is usually deferred in the 1st year of diagnosis and is associated with perioperative complications as well as increased incidence of infections in the postoperative period. Eltrombopag and romiplostim have very high complete response (CR) rates, but they are costly and need to be given for an indefinite period. Long-term remission after stopping these drugs is reported in a very small percentage of patients. Azathioprine and mycophenolate mofetil have low response and complete remission rates and take 3–6 months for optimal responses to occur.
Rituximab is a monoclonal, chimeric antibody targeted against CD20, an antigen present in B-lymphocytes. It has been found to be effective in primary and secondary ITP as a single agent and in primary ITP in combination therapy for upfront treatment of acute ITP. It is not recommended as the first-line treatment modality for ITP yet. Most studies use either low-fixed dose (100 mg) or standard dose (SD) (375 mg/m2) weekly ×4 doses along with steroids as one of the second-line treatment options for ITP.[2-4] Various studies from the West have been reported, but data on the role and place of rituximab in the management of ITP have not been studied adequately in India. We retrospectively analyzed the rituximab data of the past 8 years to see the response rates and its predictors, side effects, and long-term efficacy of rituximab in primary ITP patients.
MATERIAL AND METHODS
Type of Study: Retrospective analysis and single-centre study
Department and Institute: Department of Clinical Hematology, King George’s Medical University Lucknow
Duration: Patient data form year 2012 to 2019 were analyzed.
Study procedure
Twenty-five patients with primary ITP receiving rituximab beyond first-line treatment were retrospectively selected and analyzed. Secondary causes of thrombocytopenia and secondary ITP were ruled out by relevant investigations, including complete hemogram, peripheral smear examination, bone marrow evaluation, HCV antibody, hepatitis B surface antigen, HIV by enzyme-linked immunosorbent assay, antinuclear antibody by Hep-2 method, Coomb’s direct test, and abdominal ultrasonography/FibroScan in all patients.
Inclusion criteria
The following criteria were included in the study:
Patient with a diagnosis of primary ITP who has received rituximab as the post-first-line treatment
Patient should have followed for at least 1-month post first dose of rituximab.
Exclusion criteria
The following criteria were excluded from the study:
Patients with secondary ITP
Patient developing severe reaction to first dose of rituximab
Patients not giving informed consent.
Treatment
The patient received rituximab as the post-first-line treatment in two different dose schedules:
Low and fixed-dose rituximab – Rituximab given as intravenous infusion at doses of 100 mg weekly for 4 weeks
SD rituximab: Rituximab given at the doses of 375 mg/m2 per dose weekly for 4 weeks
All patients received dexamethasone 40 mg fixed dose on the day of each rituximab infusion irrespective of the rituximab dose.
Primary objective
Response rates at 1 month, 6 months, 12 months, and last follow-up and analysis of side effects during infusion or post-infusion.
Secondary objectives
To analyze patients achieving >50 × 109/L platelet count at 6 months and 1 year
Factors predicting response to rituximab and progression-free survival (PFS) include age, sex, interval between diagnosis to rituximab infusion, previous lines of treatment, and early response to rituximab.
Statistical analysis
The normality of the continuous variables was established, and a variable was considered normally distributed when the Z value of the skewness was found within ±3.29. The normality of the continuous variable was expressed as means ± standard deviation. The median and quartile were calculated. Categorical variables were expressed as frequencies (%). Independent samples t-tests were used to compare the means between the two groups. Chi-square tests (or Fisher exact test) were performed to test the association between two categorical variables. The Kaplan–Meier progression-free survival curves were drawn utilizing various variables. P < 0.05 was considered statistically significant. All data were analyzed by the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp).
RESULTS
Twenty-five patients receiving rituximab, post-first-line treatment for primary ITP, between year 2012 and 2019, were retrospectively analyzed in this study. Baseline characteristics of the patients are given in Table 1.
| Baseline characteristics | |
| Median age | 25 years (Q1, Q3=12,39) |
| Male: Female | 1:2 |
| Baseline mean platelet count (at the time of receiving rituximab) | 12×109±3.45×109/L |
| Previous lines of treatment | 2 (Range 1–4) |
| Previous treatment received=n(%) | Steroids-25 (100%) Azathioprin-25 (100%) Eltrombopag-5 (20%) Mycophenolate-3 (12%) Danazol-(3) 12% Splenectomy-(4) 16% IVIg-1 (4%) |
| Median duration between diagnosis and rituximab therapy | 9 months |
IVIg: Intravenous immunoglobulins
Overall response rate (ORR), CR and partial response (PR)
Two patients (8%) received rituximab as the second-line treatment, 14 (56%) as the third-line treatment, and 9 (36%) as the fourth-line treatment. Figure 1 shows the treatment pattern of all 25 patients, along with a line of treatment and responses.

- Showing recruitment of the patients for the study. Each number is consists of number of the patients. Boxes in red, yellow and green colours shows no response, partial response and complete response, respectively. (AZA: Azathioprine, DANA: Danazol, MTCO: Mycophenolate Mofetil, ELTRO: Eltrombopag, RITU: Rituximab, ROMI: Romiplostim, SPLEN: Splenectomy, IVIg: Intravenous Immunoglobuline, VIN: Vincristine).
Response rates were defined as standard criteria for response in ITP. (R) Overall, the response was seen in 18 patients (72%), while seven patients did not get any response. At the end of 2 weeks from the first dose, ORR was 52%, which increased to 80% (CR-48%, PR-32%) at 4 weeks. At 8 weeks, 50% (four out of eight) of patients achieving PR lost the response. Figure 2 shows the monitoring of the pattern of response over a period of 6 months. Considering the sustained rise of platelet counts >50 × 109/L, as one of the important response criteria in ITP studies, 40% of patients could achieve this response at 2 weeks, which increased to 60% at week 4 and then sustained at 40% at 6 months and later follow-ups. At a median follow-up of 47.6 months, 10 patients (40%) were still in CR, and 5 patients (20%) were maintaining PR with ORR of 60%. No response was seen in 7 patients (28%), and 3 patients (12%) relapsed with overall failure rates of 40%.
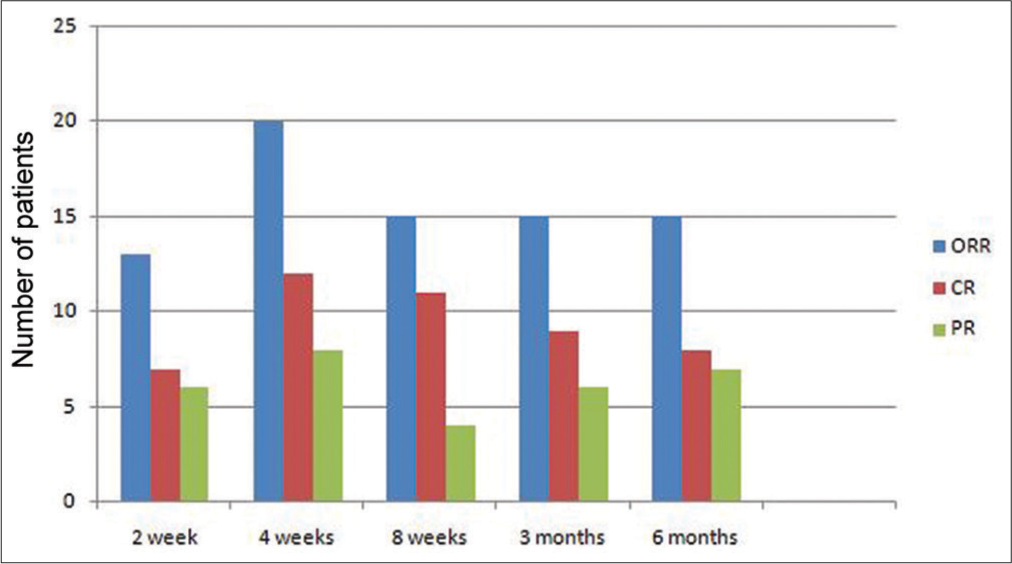
- Stacked bar chart showing dynamic changes in the response to the rituximab therapy over time in comparison to the final response (CR: Complete response, PR: Partial response, NR: No response, wk: Weeks, mn: Months).
We analyzed the factors determining the CR rates at the last follow-up, including age, sex, dose of rituximab (low dose [LD] vs. high dose), lines of treatment (≤2 lines vs. >2 lines of treatment), early (<12 months) or late (>12 months) initiation of rituximab therapy, and CR at 2 weeks and CR at 4 weeks. Age, sex, dose of rituximab, and lines of treatment did not affect the final CR rates, but there was a trend toward better CR rates in patients age> 20 years and female sex. CR at 2 and 4 weeks was significantly associated with the long-term CRs. Table 2 shows the various factors and their significance in determining the long-term CRs.
| CR (%) | PR or NR (%) | Total (%) | P-value | |
|---|---|---|---|---|
| Age | ||||
| <20 years | 1 (11.1) | 9 (56.2) | 10 (40.0) | 0.055 |
| 20–40 years | 4 (44.4) | 5 (31.2) | 9 (36.0) | |
| >40 years | 4 (44.4) | 2 (12.5) | 6 (24.0) | |
| Sex | ||||
| Female | 7 (77.8) | 10 (62.5) | 17 (68.0) | 0.661 |
| Male | 2 (22.2) | 6 (37.5) | 8 (32.0) | |
| Rituximab therapy | ||||
| Early (<6 months) | 2 (22.2) | 7 (43.8) | 9 (36.0) | 0.401 |
| Late (>6 months) | 7 (77.8) | 9 (56.2) | 16 (64.0) | |
| Line of treatment | ||||
| ≤2 lines | 7 (77.8) | 9 (56.2) | 16 (64.0) | 0.401 |
| >2 lines | 2 (22.2) | 7 (43.8) | 9 (36.0) | |
| Rituximab dose | ||||
| Low dose | 4 (44.4) | 9 (56.2) | 13 (52.0) | 0.44 |
| High dose | 5 (55.6) | 7 (43.8) | 12 (48.0) | |
| CR at 2 week | ||||
| Yes | 6 (66.7) | 1 (6.2) | 7 (28.0) | 0.003 |
| No | 3 (33.3) | 15 (93.8) | 18 (72.0) | |
| CR at 4 week | ||||
| Yes | 7 (77.8) | 5 (31.2) | 12 (48.0) | 0.033 |
| No | 2 (22.2) | 11 (68.8) | 13 (52.0) |
Fisher exact test was used. Parenthesis corresponds to the percentage of the patients. CR: Complete response, PR: Partial response, NR= No response
We analyzed PFS and the factors influencing it. Median PFS was 19 months, and at 1 and 2 years, it was 33.3% and 30.4%, respectively. Early responses, including overall response at 2 weeks, 4 weeks, early use of rituximab at <12 months, and age >20 years, had significantly better PFS. Sex, lines of treatment, and rituximab dose (SD vs. LD) did not affect the PFS. Figure 3 shows the Kaplan–Mayer curves of PFS in patients receiving rituximab.
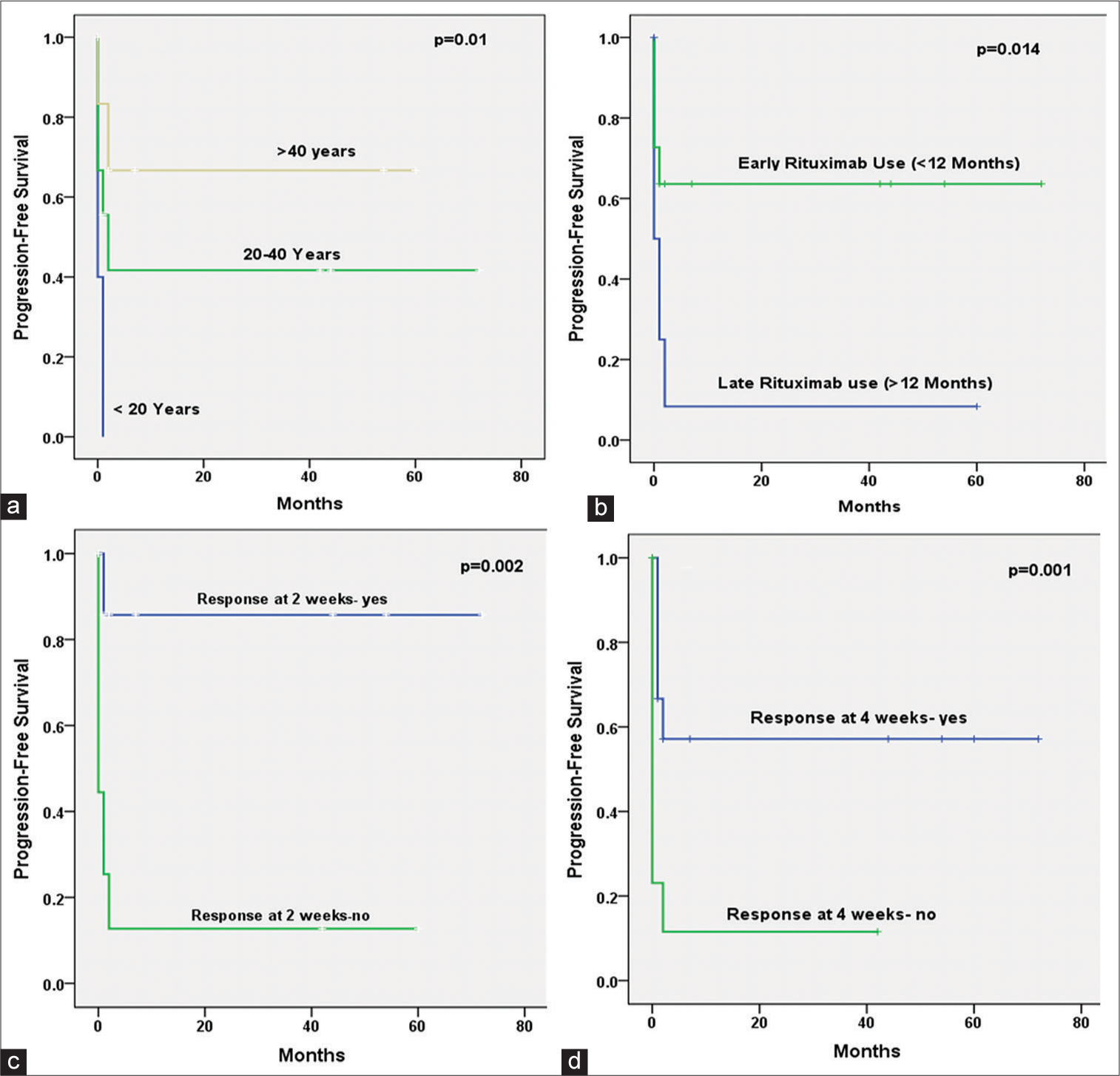
- Kaplan Meir curves show progression-free survival for an overall response (complete response and partial response). a: Age Group, b: Rituximab used <12 or ≥12 months of diagnosis; c: Response at two weeks; d: Response at four weeks. Log Rank (Mantel- Cox) test was applied and a P value <0.05 was considered significant. While, sex, Line of treatment(second line or later), Rituximab Dose (low or high), Rituximab used (within six months or later of diagnosis) were not found significant.
At the median follow-up of 47.6 months, mortality was 8%. One patient died due to refractory disease and central nervous system hemorrhage, and another patient died due to cellulites related sepsis and renal failure.
Side effects and complications of rituximab
Mild grade 1 infusion-related reaction occurred in 2 (8%) patients, and 1 (4%) patient developed left lower limb cellulites with sepsis and acute renal failure 1 week after the rituximab infusion, leading to death. Other patients did not develop any significant complications.
DISCUSSION
Initially, Stasi et al. studied the role of rituximab in 2001 in 25 cases of chronic ITP treated with 4 weekly infusions at a dose of 375mg/m2.[2] ORRs and sustained responses were 52% and 28%, respectively. No clinical or laboratory parameters predicted responses, but females and young age fared better. Miyakawa et al. studied the role of SD rituximab weekly for 4 weeks in patients with primary ITP who have relapsed or are refractory to previous lines of treatment.[3] The primary endpoint was to study the proportion of patients maintaining platelet count >50 × 109/L at 24 weeks. Out of 27 patients, 30.8% were able to maintain a platelet count above the target point. Similarly, in our study, 40% of patients were able to maintain a platelet count ≥50 × 109/L at 24 weeks.
Another important multicenter study by Dierickx et al. studied the role of rituximab in autoimmune hemolytic anemia and ITP in Belgium.[4] Out of a total of 40 patients with ITP, 12 had secondary ITP, and only 28 were primary ITP. Ninety-five per cent of patients received SD rituximab weekly for 4 weeks. ORR after the first course of rituximab was 70%, with 62.5% CR and 7.5% PR. Median PFS was 24.1 months, and at the end of 1 and 2 years, PFS was 70% and 44%. Age, sex, prior therapy, and duration from diagnosis to rituximab did not affect the outcome. Our patients also had an overall response of 80% at 4 weeks and 60% at 6 months. Median PFS was 19 months, and at 1 and 2 years, it was 33.3% and 30.4%, respectively. Early responses, including overall response at 2 weeks, 4 weeks, early use of rituximab at <12 months, and age >20 years, had significantly better PFS. Sex, lines of treatment, and rituximab dose (SD vs. LD) did not affect the PFS, although there was a trend toward better response in the female sex. At a median follow-up of 47.6 months, ORR was 60%, with CR at 40% and PR in 20% of patients, indicating that these patients are responsive to further lines of treatment, including thrombopoietin receptor agonists and splenectomy. Table 3 shows the most relevant studies with rituximab doses at 375 mg/m2 and available data of overall response, response at different time points, and response at the last follow-up.[5-10]
| Author | N | Median age (years) | M: F | Phase of ITP | ORR (%) | 6 months | 12 months (%) | Last follow-up |
|---|---|---|---|---|---|---|---|---|
| Medeot et al.[5] (2008) | 26 | 55 | 4:1 | chronic phase (most) | 69 | NA | 55 | 35% at MFU 57 months |
| Patel et al.[6] (2012) | 72 | 39 | 65:35 | Chronic phase (most) | 57 | NA | 38 | 21% at 5-year FU |
| Mahévas et al.[7] (2013) | 61 | 52 | 64:36 | Persistent (26%) Chronic (56%) |
54 | NA | 36% | 31% at a median follow-up of 36 months |
| Khellaf et al.[8] (2014) | 173 | 51 | 64:36 | Chronic (56%) | 62 | 80 | 62% | 38% at 36 months, last follow-up NA |
| Ghanima et al.[9] (2015) | 55 | 46 | 73 :27 | 24% PD 44% CP |
73 | 60 | 45% | 24% at 78 weeks FU |
| Marangon et al.[10] (2017) | 103 | 46 | 59:41 | 55% | 55% | NA | NA | 21% at 96 months |
ITP: Immune thrombocytopenia, MFU: Median follow up, FU: Follow up, NA: Not available, PD: Persistent Disease, CP: Chronic phase, ORR: Overall response rates
LD rituximab has been studied as the first-line treatment along with high-dose dexamethasone as well as post-first-line treatment of ITP. A study by Zhou et al. evaluated the role of rituximab and high-dose dexamethasone as the first-line treatment options with 100% ORR at day 28 and responses of 83.3% and 61.5% at 6 months and 12 months, respectively, with adverse event incidence of 11.1%.[11] Zaja et al. studied LD rituximab in 48 patients, mostly chronic ITP, with ORR of 60.5% and CR rates of 39.5%. Overall responses at 12 and 24 months were 61% and 45%, respectively.[12] In another study, he compared LD rituximab with SD rituximab. Overall response and response at the last follow-up was 52% and 23% in the LD rituximab arm compared to 66% and 35% in the SD rituximab arm. Contrary to this, Gracie et al. found similar results in LD rituximab versus SD rituximab with ORR of 61% versus 64% at 6 months and 50% versus 40% at 3 years follow-up, respectively.[13] In our study, 13 patients received LD (100 mg fixed weekly dose) rituximab with ORR-47.1% compared to 52.9% in patients receiving SD rituximab (P = 0.67). Recently, a meta-analysis by Li et al. showed that pooled ORR was 63% and pooled CR was 44%.[14] This analysis included 329 patients from nine studies. Thirtyone patients had side effects, and 30 of them were mild to moderate, no deaths were reported. This clearly indicates that LD rituximab is an effective and safe option.
Predictors of response
Several studies have shown the association of age and gender with the outcome. A study by Stasi et al. showed that women and young patients have better responses.[2] Bussel et al. showed that women with childbearing age and duration of disease <2 years have better responses almost comparable to splenectomy.[15] Another study with similar treatment protocol showed better responses in adolescent females with disease duration <1 year.[16] In our study, we could not demonstrate any significance of age and gender in predicting outcomes. This is supported by many other studies where no correlation was proven between age, gender, and response rates.[6,17,18] This is in contrast to our study, where we found much better responses and better PFS in females between 20 and 40 years of age, similar to Bussel et al. and Stasi et al. We also found that CRs at 2 and 4 weeks predict higher long-term CR, and overall response at 2 and 4 weeks predicts better PFS. Similar observations have been reported in other studies.[2,15]
Adverse events
Infusion-related adverse events are most common and reported to vary between 15 and 60%.[6,8-19] This occurs due to immediate cytokine release. We found such adverse events in 8% of cases. No viral reactivation was noted in any of our patients. One patient expired post-rituximab therapy due to left lower limb cellulitis and related sepsis.
Study limitations
Retrospective nature of the study and low number of patients are the major limitations of our study.
CONCLUSION
With our study results and available literature, it is clear that rituximab still has a very important role in the management of persistent and chronic ITP. Overall response is up to 80%, and long-term response ranges between 30% and 40%. Early response at 2 and 4 weeks, early use of rituximab (<1 year), and age more than 20 years correlate with significantly better outcomes. The dose of rituximab (SD vs. LD) and prior lines of treatment do not affect the outcome. Side effects associated with rituximab are mainly infusion-related reactions, and these are easily manageable. Due to the definite duration of treatment and lower cost of therapy, rituximab will remain a feasible post-first-line treatment option in the near future.
Authors’ contributions
SPV and PP: Conceptualization, writing and supervision; AKT and BS: Proofreading; AS and DPV: Writing and discussion;ASC and RK: Investigations and draft preparation; MO: Statistical analysis.
Ethical approval
Institutional Review Board has waived the ethical approval for this study. Approval Waiver number 906/Ethics/2021 dated 5th August 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an International working group. Blood. 2009;113:2386-93.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab chimeric anti-CD20 monoclonal antibody treatment for adults with chronic idiopathic thrombocytopenic purpura. Blood. 2001;98:952-7.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of rituximab in Japanese patients with relapsed chronic immune thrombocytopenia refractory to conventional therapy. Int J Hematol. 2015;102:654-61.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab in auto-immune haemolytic anaemia and Immune thrombocytopenic purpura: A belgian retrospective multicentric study. J Intern Med. 2009;266:484-91.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab therapy in adult patients with relapsed or refractory immune thrombocytopenic purpura: Long-term follow-up results. Eur J Haematol. 2008;81:165-9.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119:5989-95.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of rituximab given at 1,000 mg on days 1 and 15 compared to the standard regimen to treat adult immune thrombocytopenia. Am J Hematol. 2013;88:858-61.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of rituximab in adult immune thrombocytopenia: Results from a prospective registry including 248 patients. Blood. 2014;124:3228-36.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): A multicentre, randomized, double-blind, placebo-controlled trial. Lancet. 2015;385:1653-61.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab in immune thrombocytopenia: Gender, age, and response as predictors of long-term response. Eur J Haematol. 2017;98:371-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical efficacy and safety of high-dose dexamethasone plus low-dose rituximab as first-line therapy in newly diagnosed primary immune thrombocytopenia. Indian J Hematol Blood Transfus. 2019;35:507-12.
- [CrossRef] [PubMed] [Google Scholar]
- Low-dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol. 2010;85:329-34.
- [CrossRef] [PubMed] [Google Scholar]
- Comparsion of standard-and low-dose rituximab in primary immune thrombocytopenia (ITP): Data from the UK ITP registry In: Abstract presented at: The 2018 International conference of EHA. 2018.
- [Google Scholar]
- The efficacy and safety of low-dose rituximab in immune thrombocytopenia: A systematic review and meta-analysis. Platelets. 2019;30:690-7.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab and three dexamethasone cycles provide responses similar to splenectomy in women and those with immune thrombocytopenia of less than two years duration. Haematologica. 2014;99:1264-71.
- [CrossRef] [PubMed] [Google Scholar]
- Gender and duration of disease differentiate responses to rituximab-dexamethasone therapy in adults with immune thrombocytopenia. Am J Hematol. 2016;91:907-11.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of rituximab in primary immune thrombocytopenia: An analysis of adult pretreated patients from everyday hematological practice. Int J Hematol. 2012;96:594-9.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab in the management of chronic immune thrombocytopenic purpura: An effective and safe therapeutic alternative in refractory patients. Ann Hematol. 2006;85:400-6.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125:232-9.
- [CrossRef] [PubMed] [Google Scholar]







