Translate this page into:
Analysis of early molecular response at 3 months in predicting overall response in newly diagnosed patients with chronic myeloid leukemia on imatinib
*Corresponding author: Asif Iqbal, Department of Medical Oncology, Dr. B. Borooah Cancer Institute, Guwahati, Assam, India. driqbal85.ai@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Iqbal A, Nath UK, Bhattacharyya M, Nag A, Ray SS. Analysis of early molecular response at 3 months in predicting overall response in newly diagnosed patients with chronic myeloid leukemia on Imatinib. J Hematol Allied Sci 2022;2:32-8.
Abstract
Objectives:
This study aimed to study the correlation between Breakpoint Cluster Region- ABelson Leukemia virus 1 transcript levels at 3 months with the treatment responses at 6 and 12 months in patients on imatinib. Around 30% of patients with chronic myeloid leukemia (CML) might have treatment failure with the first-line tyrosine kinase inhibitors (TKI). Patients with a “warning response” at 3 months can continue therapy with the same TKI while monitoring for disease progression. However, newer pieces of evidence suggest that patients who fail treatment with imatinib do have suboptimal responses in the early time points, and hence, 1st 3-month assessment might be a useful indicator for future treatment failure.
Material and Methods:
It is a single-center prospective observational study involving 60 treatment-naive consecutive patients with CML-chronic phase who attended Hematology Outpatient Department at IHTM, Kolkata. Treatment responses were assessed by cytogenetics and BCR-ABL1 transcript levels by real-time quantitative polymerase chain reaction at 3 monthly time points.
Results:
About 51% and 70.2% of the study participants achieved complete cytogenetic response at 6 and 12 months, respectively. About 74% of the participants had achieved early molecular response (EMR) at 3 months. The failure rates of cytogenetic responses were 13% and 20% at 6 and 12 months, respectively. Patients who failed to achieve EMR at 3 months had higher failure rates at 6 months. The major, warning and failure of molecular responses at 6 and 12 months were found to be 15%, 25%, and 9%, and 34%, 39%, and 27%, respectively. The analyses showed that patients who failed to achieve EMR at 3 months are also more likely to have the failure of molecular response at 12 months, with a statistical significance of P < 0.01. Failure of EMR at 3 months also correlated with failure of overall responses (both cytogenetic and molecular at 12 months) with a statistical significance of P = 0.006. When followed up, there was a progression of disease in three including a death in the suboptimal response group.
Conclusion:
Our patients had inferior treatment responses to imatinib than that observed in the previous studies. The majority have baseline fibrosis of the marrow and splenomegaly at presentation which might contribute to adverse outcomes. The molecular response at 3 months was found to be a consistent and powerful indicator of treatment responses at later time points.
Keywords
Chronic myeloid leukemia
BCR-ABL
Early response assessment
Early molecular response
INTRODUCTION
Chronic myeloid leukemia (CML) is a malignant hematopoietic condition classified under “Myeloproliferative neoplasms” that is characterized by the (9;22) chromosomal translocation that produces the Philadelphia (Ph) chromosome.[1] It was the first malignancy and one of the few discovered to date, which was identified to be caused by a unique chromosomal translocation.[2] Due to this chromosomal translocation, an oncogenic BCR-ABL fusion protein containing a constitutively active tyrosine kinase is produced which causes excessive and unrestricted proliferation of myeloid cells.
Over the past two decades, the management of CML has evolved manifold. In those times, the outcomes were poor even after treatment with curative potentials such as allogeneic stem cell transplantation and recombinant interferon-alfa. After tyrosine kinase inhibitors (TKIs) were discovered, a cure for CML became a possibility.[3] A quarter of patients, however, fail to respond or lose initially acquired response to imatinib. European leukemia net (ELN) laid down recommendations to address these patients who responded suboptimally to imatinib.[4] Many of these patients respond and attain long-term remission with the use of 2nd line TKIs, that is, dasatinib and nilotinib.[5,6]
In light of changing treatment paradigm, the ELN expert panel updated treatment recommendations in 2013[7] and then in 2020.[8] Responses to treatment are assessed by quantitation of BCR-ABL1 by real-time polymerase chain reaction (qPCR) and/ or cytogenetics at 3, 6, and 12 months. Responses are classified as “optimal response,” and “failure.” A suboptimal response not accounting for “failure” was termed as a “warning response.” Early molecular response (EMR) at 3 months, defined as ≤10% BCR-ABL1 International Scale transcript level, was found to correlate with long-term outcomes including progression-free survival and overall survival.[9-11] This and other pieces of evidence including the International Randomized Study of Interferon and STI571 (Imatinib) follow-up study also showed that early responses to imatinib predict the overall responses.[12]
MATERIAL AND METHODS
Specific objective
The objective of this study was to study the correlation of EMRs at 3 months with the cytogenetic and molecular responses at 6 months and 12 months after starting first-line therapy with imatinib
Study setting
Study area
This study was Institute of Hematology and Transfusion Medicine, Medical College and Hospital, Kolkata.
Study population
This study was on newly diagnosed patients of the CML-chronic phase (CML-CP).
Timeline
This study was January 2015–December 2015.
Study design
This study was a prospective observational study.
Inclusion criteria
The following criteria were included in the study:
All newly diagnosed cases of CML patients being initiated on Imatinib therapy
Documented chronic phase CML fulfilling the criteria as below:
<10% blasts in peripheral blood and bone marrow and <20% basophils in the peripheral blood
No evidence of extramedullary leukemic involvement except for hepatosplenomegaly
Ability to provide written informed consent before initiation of the study.
Exclusion criteria
The following criteria were excluded from the study:
Patients without confirmed Ph chromosome at diagnosis
Accelerated phase/Blast crisis
Patients not attaining complete hematologic response (CHR) at 3 months of starting imatinib therapy
Treatment with TKI before study entry
Any medical treatment for CML before study entry for longer than 2 weeks
Loss of imatinib treatment days of more than 30 days in the first 6 months and 45 days in 1 year due to any cause
Patients who are non-compliant with response assessments are defined as failing to perform 1st response assessment (3 months) beyond 15 days, 2nd response assessment (6 months) beyond 30 days, and 3rd response assessment (12 months) beyond 45 days of schedule, respectively.
Sample size
This study was 60 cases.
Parameter studied
Initially, in the first outpatient department (OPD) visit of patients with suspicion of CML, the following baseline parameters were recorded:
Spleen size below costal margins in CMS
Total leukocyte count
Platelet count
Blast percentage
Basophil percentage, and
Eosinophil percentage.
The count in the OPD was performed on the Sysmex KX21 counter.
If the patient’s presenting Total Leukocyte Count was more than 1 lakh/μl, they received Cap. Hydroxyurea 1 g/day and if it was more than 2 lakh/μl, they received Cap. Hydroxyurea 2 g/day was initiated with plenty of oral fluid and allopurinol. Hydroxyurea was initiated in patients only after the Bone marrow aspirates were obtained for cytogenetic analysis.
Bone marrow aspiration, biopsy, conventional cytogenetics (if required fluorescent in situ hybridization [FISH]) from the bone marrow in heparin vacutainers and real-time PCR (RT-PCR) for BCR-ABL from peripheral blood in Ethylene diamine tetra acetic acid vacutainers were performed on the initial diagnostic samples.
The patients were started on imatinib 400 mg daily only after the RT-PCR for BCR-ABL report become available and was positive, and then, Hydroxyurea if started earlier was stopped.
Then, a complete blood count with peripheral smear and clinical evaluation for spleen size and side effects were performed every 2 weeks till 3 months and then every month till 1 year.
RQ-PCR for BCR-ABL transcript level was performed at 3 and 12 months of starting therapy and bone marrow aspiration for cytogenetics was performed at 6 and 12 months of starting therapy. Few patients have been followed up for more than 1 year.
Study tools
This study was a clinical Pro forma and Laboratory progress sheet.
Plan of study
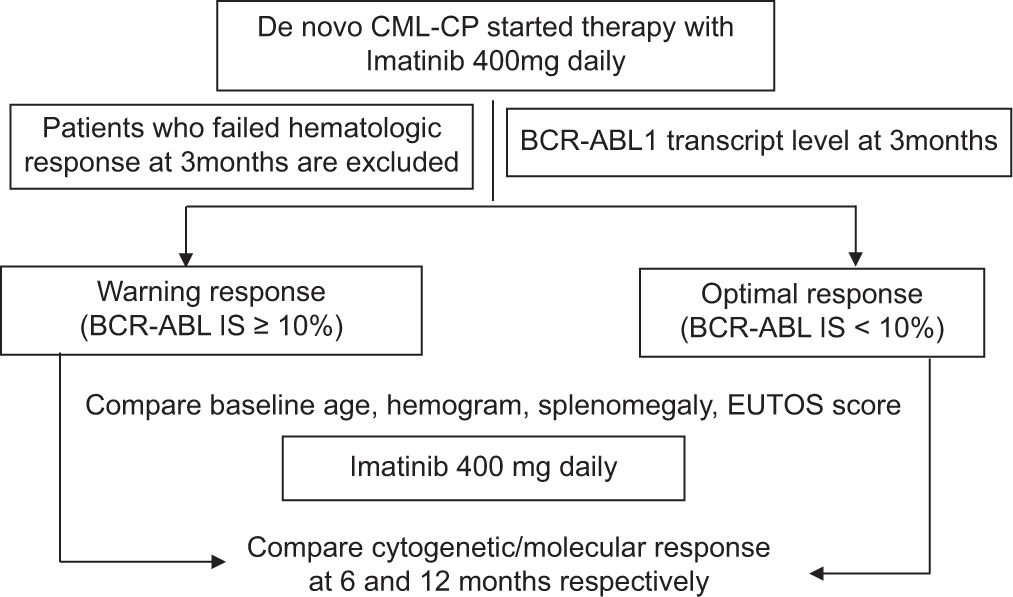
Ethical issues
The study was conducted as per the Indian Council of Medical Research Guidelines for Research on Human subjects, Schedule Y (Amended Version 2013), Declaration of Helsinki (Fortaleza, Brazil, October 2013), and other applicable guidelines.
Ethical committee approval
This study was started after written approval was obtained from the Institutional Ethics Committee (IEC) of Medical College and Hospital, Kolkata.
Definitions of response to imatinib therapy
-
CHR:
No signs or symptoms of disease (splenomegaly)
WBC <10 × 109/L with normal differentials
Platelets <450 × 109/L
-
Cytogenetic responses:
Complete: 0% Ph + metaphases
Major: 1–35% Ph + metaphases
Minor: 36–65% Ph + metaphases
Minimal: 66–95% Ph + metaphases
None: >95% Ph+ metaphases.
The molecular response is expressed and reported as the transcript value of BCR-ABL1 on a log scale. A decrease of log 3 is termed major molecular response (MMR) and a decrease more than log 4 is termed deep molecular response.
RESULTS AND OBSERVATIONS
During the study period of 12 months (January 2015– December 2015), we recruited 60 patients. Seven patients were excluded on grounds of various exclusion criteria (three lost to follow-up before the 1st response assessment, two had no Ph chromosome in the cytogenetic study, one presented with accelerated phase, and one patient died of an unrelated cause). Study allocation was done to 53 patients. Out of the allocated patients, only 47 patients completed the study. The rest were excluded from the study due to not achieving Complete hematologic response(1), non-compliance with drug/response assessment (3), and poor tolerance to Imatinib (2).
Baseline characteristics of the patients
The baseline characteristics are described in [Table 1].
| Variable | n (%) |
|---|---|
| Total patients in CML-CP | 53 |
| Patients under evaluation | 47 |
| Age-median years (range) | 35 (17–67) |
| Gender | |
| Male | 32 |
| Female | 15 |
| Ratio | 2.1:1 |
| Spleen size | |
| Median in cms (range) | 8 (3–18) |
| Spleen size > 7 cm | 30 (56.6) |
| Basophils – Median in % (range) | 5 (2–12) |
| Eosinophils – Median in % (range) | 5 (2–15) |
| Blasts – Median in % (range) | 2 (0–9) |
| Hemoglobin | |
| Median in g/dl (range) | 8.8 (5.4–12.7) |
| Hb < 7 g/dl | 6 (11.3) |
| Platelet count in 109/L | |
| Median (range) | 252 (61–1199) |
| Total leukocyte count in 109/L | |
| Median (range) | 86 (10.4–556) |
| EUTOS score | |
| High (>87) | 16 (34) |
| Low(<87) | 31 (66) |
| Cytogenetics at diagnosis | 47 |
| Conventional karyotyping | 42 |
| FISH | 5 |
| Other cytogenetic abnormalities at diagnosis | 9 (21.4) |
| Total number of patients who received hydroxyurea | 18 (34) |
| The average number of days on Hydroxyurea | 12 days |
| Patients attaining CHR (among all evaluable patients up to 1st 3 months) | 48/49 (97.9%) |
| Patients with the progression of disease with therapy | 2/48 (4.1%) |
Cytogenetic abnormalities at diagnosis
Among the 47 patients, conventional karyotyping was done in 42 patients. The rest of the five patients had non-dividing cells and were diagnosed with FISH. Out of the 42 patients, 33 had classic Ph abnormalities, and nine showed additional cytogenetic abnormalities.
Other parameters
The peripheral blood blast percentage ranges from 0% to 9%, with a median of 2%. Forty patients (86.9%) had blast percentages below 5%. The bone marrow blast percentage ranged from 2% to 9%, with a median of 5%. There was a strong concordance among circulating and marrow blast percentages.
All the patients were also evaluated for myelofibrosis using reticulin and trichrome staining of the trephine biopsy. Grading was done by the World Health Organization grading of myelofibrosis (0–3). The median grade of bone marrow fibrosis present in these patients was Grade 1. Significant fibrosis (Grade 2–3) was present in 19 (40.4%) with four patients having Grade 3 myelofibrosis. The absence of myelofibrosis was noted in 6 (12.7%) patients.
Response assessments of the study patients
CHR
Out of the 53 patients allocated to the study, one patient did not achieve CHR at 3 months, and three patients were intolerant/non-compliant with imatinib before the end of 12 weeks of therapy. The median duration to attain CHR in the rest was 6 weeks (2–12 weeks).
EMR at 3 months
The 1st assessment for a molecular response was done on 49 patients. However, because two patients were later left out of the study, the data have been computed on the rest of the 47 patients [Figure 1].

- BCR-ABL1 transcript level at 3 months.
Early cytogenetic response at 6 months
The 1st cytogenetic response assessment by conventional karyotyping of the bone marrow aspirate was done at 6 months. Twenty-four patients (51%) achieved complete cytogenetic response (CCyR) and six patients (12.7%) did not achieve a partial cytogenetic response (PCR), defined as failure of response.
Response assessments at 12 months
Molecular response at 12 months
Out of 47 study patients, six patients failed the 6-month response assessment. At 12 months, a molecular response assessment was done on 41 patients. Fourteen patients (34.1%) achieved MMR. Eight out of them had no detectable transcripts in qPCR (undetectable leukemia or complete molecular response). However, 11 patients (26.8%) had a failure in molecular response at 12 months.
The BCR-ABL1 transcript level and the distribution of the 12-month molecular response of the patients are shown in [Figure 2].

- BCR-ABL1 transcript level at 12 months.
Cytogenetic response at 12 months
Out of 41 patients on whom a cytogenetic response was assessed at 12 months, 33 (80.4%) achieved CCyR. The rest of the eight patients (19.5%) had a failure of cytogenetic response at 12 months.
The responses to imatinib 400 mg/d at the different timelines in the group of study patients who have achieved a CHR at or before 12 weeks and have been compliant with the drug and the monitoring schedules are shown in [Table 2] below.
| Optimal | Warning | Failure | |||
|---|---|---|---|---|---|
| 3 months | 35 | 12 | 47 | ||
| 6 months | 24 | 17 | 6 | 47 | |
| 12 months | 14 | 15 | 12 | 41 | |
| Total failure of therapy | 18 |
At the end of 12 months of imatinib therapy in 47 patients with CML, 14 (29.7%) achieved optimal responses in all the timelines. Fifteen patients (31.9%) had warning responses, while 18 patients (38.2%) had a failure of therapy and had to be switched to alternative therapy before or at 12 months.
Result of outcomes in patients in two response arms
The consort diagram below depicts the study participants in the two arms according to their responses at 3 months, namely:
Arm A: Optimal molecular response at 3 months
Arm B: Warning molecular response at 3 months.

Comparisons of response assessments to therapy of the two arms in 6 months
The patients of the two response arms were followed up with imatinib 400 mg/d without any therapy modifications.
When comparisons were made for warning cytogenetic responses at 6 months for these two groups of patients, the association was not found to be significant (P = 0.35). However, when the comparisons were made for the failure of cytogenetic responses at 6 months, it was found to be statistically significant with P = 0.0017.
Comparisons of response assessments to therapy of the two arms at 12 months
The patients who failed therapy at 6 months were scheduled for alternative therapy options (2nd line TKI or high dose Imatinib) and were excluded from the present study. The rest (n = 41) were followed up with imatinib 400 mg/d and responses were analyzed with cytogenetics and qPCR at 12 months.
[Table 3] shows the cytogenetic, molecular, and overall responses at 12 months in these two groups of patients.
| Cytogenetic responses at 12 months | Arm A | Arm B | |
|---|---|---|---|
| Optimal | 29 | 4 | 33 |
| Failure | 5 | 3 | 8 |
| Total | 34 | 7 | 41 |
| Molecular responses at 12 months | |||
| Optimal | 14 | 0 | 14 |
| Warning | 15 | 1 | 16 |
| Failure | 5 | 6 | 11 |
| Total | 34 | 7 | 41 |
| Overall responses at 12 months | |||
| Optimal | 14 | 0 | 14 |
| Warning | 14 | 1 | 15 |
| Failure | 6 | 6 | 12 |
| Total | 34 | 7 | 41 |
When comparisons were made for optimal/failure cytogenetic responses at 12 months for these two groups of patients, the association was not found to be significant (P = 0.086). The optimal and failed molecular responses were compared and found to be significant (P = 0.0055). When comparisons were made for the overall outcomes at 12 months in the two response arms, the association was found to be significant for the occurrence of failure of therapy in Arm B (P = 0.006).
Follow-up
The patients in the study were followed up for 14–20 months (median = 16 months). One patient in Arm B who had failed to respond at 12 months died of progressive disease. However, three other patients who had warning responses at 12 months attained MMR at 18 months and one of them had an undetectable disease. The rest are doing well until their last visit.
DISCUSSION
The present study comprised 47 newly diagnosed CML-CP patients. All of them received Tab Imatinib 400 mg daily. It was a prospective study aimed to find out a correlation between an EMR at 3 months with responses at 6 and 12 months. The median age of patients in our study was 35 years.
EMR at 3 months
Only one patient allocated to the study did not achieve CHR. Molecular responses at 3 months were performed on 49 patients. Thirty-five patients (74.4%) out of the analyzed, 47 study patients achieved optimal EMR at 3 months. The 3 months response rate in the patients of the present study was comparable to most of the studies listed in western and Indian literature.
Cytogenetic response at 6 months
Twenty-four patients (51%) achieved CCyR (optimal response). Six patients (12.7%) patients failed to achieve PCyR and were termed as a failure of response. When compared with the previous studies, CCyR was observed at 6 months in the range of 50–70% of participants.
Responses at 12 months
The 12-month responses of patients in our study and various other studies with Imatinib 400/d have been presented in a tabular form below.
The molecular response rates at 12 months were unfavorable when compared to the patients in most of the previous studies [Table 4].[13-19] One-third of our patients had an optimal molecular response at 12 months and over a quarter had a failure at the same time point. The data were analyzed by excluding those patients who failed responses at time points before 12 months highlighting a poor response to imatinib 400 mg in our group of patients. When the observation is compared to other contemporary Indian and western data, the rates of MMR at 12 months were found to be 5–15% inferior. When analyzed concerning the molecular responses at 3 months, the patients who had warning/suboptimal molecular responses had higher rates of failure at 12 months. This observation tallied with the previous other studies which demonstrated poor 12-month responses and higher disease progression in patients with suboptimal 3-month responses to Imatinib.[9-12]
| Study | Journal and year | CCyR at 12m | MMR at 12m |
|---|---|---|---|
| O’Brien et al. (IRIS) | NEJM, 2003 | 73.2% | ____ |
| Kantarjian et al. | Blood, 2006 | 75% | ____ |
| Druker et al. | NEJM, 2006 | 69% | ____ |
| Hughes et al. | Blood, 2010 | 70% | 41% |
| Cardama et al. | Blood, 2009 | 66% | 44% |
| Gugliotta, et al. | Blood, 2012 | 65% | 39% |
| Cortes, et al. | Clin Can Res, 2006 | 72% | 45% |
| Our study | 80%* | 34% |
CONCLUSION
The present study is a prospective observational study of 47 consecutively enrolled newly diagnosed patients with CML-CP aimed to find the cytogenetic and molecular response rates at 6 and 12 months after initial treatment with Imatinib 400 mg daily and to compare the results among the patients who achieved optimal EMR with those who did not.
About 50% of the patients achieved CCyR at 6 months, and 70% achieved CCyR at 12 months. The rates of failure of cytogenetic responses were 13% and 20% at 6 and 12 months, respectively. These rates were inferior to what was observed in the previous studies.
The major, warning and failure of molecular responses at 12 months in our group of patients were found to be 34%, 39%, and 27%, respectively. These observations were also inferior to the previously reported results. The response rates at 6- and 12-month time points were inferior for the patients who did not achieve optimal molecular response (EMR) at 3 months. When these two groups were compared for the overall response at 12 months, the findings were statistically significant.
To conclude
A greater number of our patients have bone marrow fibrosis and splenomegaly at presentations
Both molecular and cytogenetic responses to Imatinib were inferior in our patients to previously reported results
The EMR at 3 months was found to be a robust indicator of responses at later time points and failure of therapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Conventional and molecular cytogenetic basis of hematologic malignancies In: Hematology Basic Principles and Practice (6th ed). Ch. 54. Philadelphia, PA: Saunders/ Elsevier; 2013.
- [Google Scholar]
- A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132:1497.
- [Google Scholar]
- Indications for imatinib mesylate therapy and clinical management. Oncologist. 2004;9:271-81.
- [CrossRef] [PubMed] [Google Scholar]
- Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809-20.
- [CrossRef] [PubMed] [Google Scholar]
- Dasatinib: A tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Ther. 2007;29:2289-308.
- [CrossRef] [PubMed] [Google Scholar]
- Nilotinib: A second-generation tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia. Clin Ther. 2008;30:1956-75.
- [CrossRef] [PubMed] [Google Scholar]
- European leukemianet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-84.
- [CrossRef] [PubMed] [Google Scholar]
- European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966-84.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-8.
- [CrossRef] [PubMed] [Google Scholar]
- Initial molecular response at 3 months may predict both response and event-free survival at 24 months in imatinib-resistant or intolerant patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase treated with nilotinib. J Clin Oncol. 2012;30:4323-9.
- [CrossRef] [PubMed] [Google Scholar]
- Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML) Leukemia. 2012;26:2096-102.
- [CrossRef] [PubMed] [Google Scholar]
- The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic-phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118:4541-6.
- [CrossRef] [PubMed] [Google Scholar]
- The EUTOS identifies chronic myeloid leukemia with poor prognosis treated with imatinib first or second line. Leuk Res. 2012;36:e209-10.
- [CrossRef] [PubMed] [Google Scholar]
- Tyrosine kinase inhibitor usage, treatment, outcome, and prognostic scores in CML: Report from the population-based Swedish CML registry. Blood. 2013;122:1284-92.
- [CrossRef] [PubMed] [Google Scholar]
- Combining BCR-ABL1 transcript levels at 3 and 6 months in chronic myeloid leukemia: Implications for early intervention strategies. Blood. 2013;121:2739-42.
- [CrossRef] [PubMed] [Google Scholar]
- Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123:494-500.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of cytogenetic response at 6 months of therapy in global survival of patients with chronic myeloid leukemia treated with imatinib in the south of Brazil. Blood. 2012;120:4453.
- [CrossRef] [Google Scholar]
- Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408-17.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of early dose intensity on cytogenetic and molecular responses in chronic-phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood. 2008;112:3965-73.
- [CrossRef] [PubMed] [Google Scholar]






