Translate this page into:
Association between ABO blood groups and risk of COVID-19 infection: An umbrella review
*Corresponding author: Dorra Parv, Department of Clinical Psychology, Faculty of Medicine, Iranshahr University of Medical Sciences, Iranshahr, Iran. dorraparv@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Parv D, Shahnavazi A. Association between ABO blood groups and risk of COVID-19 infection: An umbrella review. J Hematol Allied Sci. 2024;4:3-10. doi: 10.25259/JHAS_26_2023.
Abstract
Numerous primary studies, systematic reviews, and meta-analyses have been conducted to examine the association between ABO blood groups and susceptibility to coronavirus disease 2019 (COVID-19) infection. The findings, however, are preliminary and contentious. As a result, the following umbrella review examines the relationship between ABO blood groups and the risk of COVID-19 infection. From December 9, 2020, to December 29, 2020, relevant articles were searched using Google Scholar, Google, and Cochrane systematic review databases. After eliminating duplicates and screening records based on article titles, abstracts, and full texts, four full article texts met the inclusion criteria. The data were analyzed using a narrative approach. This umbrella review suggests that blood group A may be a risk factor for COVID-19 infection and blood group O may be a protective factor; however, all studies included in this umbrella review reported significant heterogeneity across primary studies, which may explain inconsistent and discordant findings regarding the relationship between ABO blood groups and risk of COVID-19 infection, severity, and mortality outcomes, thereby limiting the findings. Thus, additional methodologically rigorous and experimental research and prospective cohort studies are warranted.
Keywords
ABO blood groups
Coronavirus disease 2019
Umbrella review
INTRODUCTION
SARS-CoV-2, a novel coronavirus that causes the new infectious Coronavirus Disease-2019 (COVID-19), is rapidly spreading throughout the world and was recently declared a pandemic by the World Health Organization (WHO).[1-3] As of 5:22 p.m. CEST on August 20, 2021, the WHO had confirmed 209,876,613 cases of COVID-19 worldwide, including 4,400,284 deaths.[4]
Recently, several studies discovered a link between the ABO blood group and COVID-19 morbidity and mortality.[2] In addition, epidemiology has identified risk factors for SARS-CoV-2 (COVID-19) infection, including age, gender, chronic disease, and smoking.[2,5-7] The findings, however, are preliminary and controversial. Researchers’ findings regarding the highest risk of infection, specifically the association between ABO blood groups and the risk of COVID-19 infection, are varied. Most researchers report that individuals with blood type A face the most significant risk of infection with COVID-19, while others report that individuals with blood type B face the greatest risk of infection. Although some researchers found no correlation between blood types and COVID-19 severity or mortality, the majority of studies discovered that blood types A and AB were associated with an increased risk of severe illness or death. In contrast, blood type O was associated with a protective effect against death or severe outcomes.[8] To this end, the current umbrella review was conducted using a narrative approach to synthesize the systematic reviews and meta-analyses regarding the association between the ABO blood group and COVID-19 infection.
MATERIALS AND METHODS
This review was conducted per the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).[9]
Data sources and search strategy
The authors independently conducted a systematic electronic search in the Google Scholar, Google, and Cochrane databases of systematic reviews, without regard for the country of origin or publication date, using the search terms “ABO blood group, COVID-19, Coronavirus, SARS-COVID-2, Systematic Review, and Meta-Analysis.” The reference lists of the final included articles were manually screened to identify related articles to ensure a thorough literature search.
Study selection
Inclusion criteria were as follows
Systematic reviews, with or without meta-analysis, of primary studies that (1) investigate the association between COVID-19 infection and ABO blood group; (2) provide original data; (3) compare COVID-19 cases to healthy controls; and (4) provide at least one COVID-19 infection or morbidity outcome.
We excluded narrative reviews, systematic reviews, and meta-analysis protocols, letters to the editor, and articles that were not peer-reviewed.
The authors selected studies independently. After removing duplicates, records were screened using their titles and abstracts. Finally, the full text of the remaining articles was read, and final eligible articles were selected utilizing inclusion and exclusion criteria.
Data extraction
The authors extracted key data independently based on participant characteristics, study information, and the primary outcome or overall conclusion of each included study regarding the association between COVID-19 infection and the ABO blood group (A, B, AB, or O) using a data extraction form developed by the authors.
Quality assessment
The AMSTAR assessment tool was used to determine the study’s quality.[10] To assist policymakers and clinicians, the Canadian Agency for Drug and Technologies in Health classifies the AMSTAR quality assessment into three categories: High (9–11), medium (5–8), and low (0–4).[10] The authors independently assessed the quality of the studies included in the study. Disagreements were resolved through discussion.
Statistical analysis
The authors synthesized the data from the included studies using a narrative method.
RESULTS
Database and search strategy
The Google Scholar, Google, and Cochrane Databases of Systematic Reviews literature searches returned 140 records. After removing duplicates, the authors screened 115 titles and abstracts, excluding 106 records that did not meet the inclusion criteria. Finally, the authors conducted a thorough review of the remaining studies’ full texts. Four studies remained after applying the inclusion and exclusion criteria and were included in the present or this umbrella review. In addition, the reference lists of the four studies included were screened for pertinent articles. Figure 1 illustrates the PRISMA flow diagram[11,12] for the literature search.
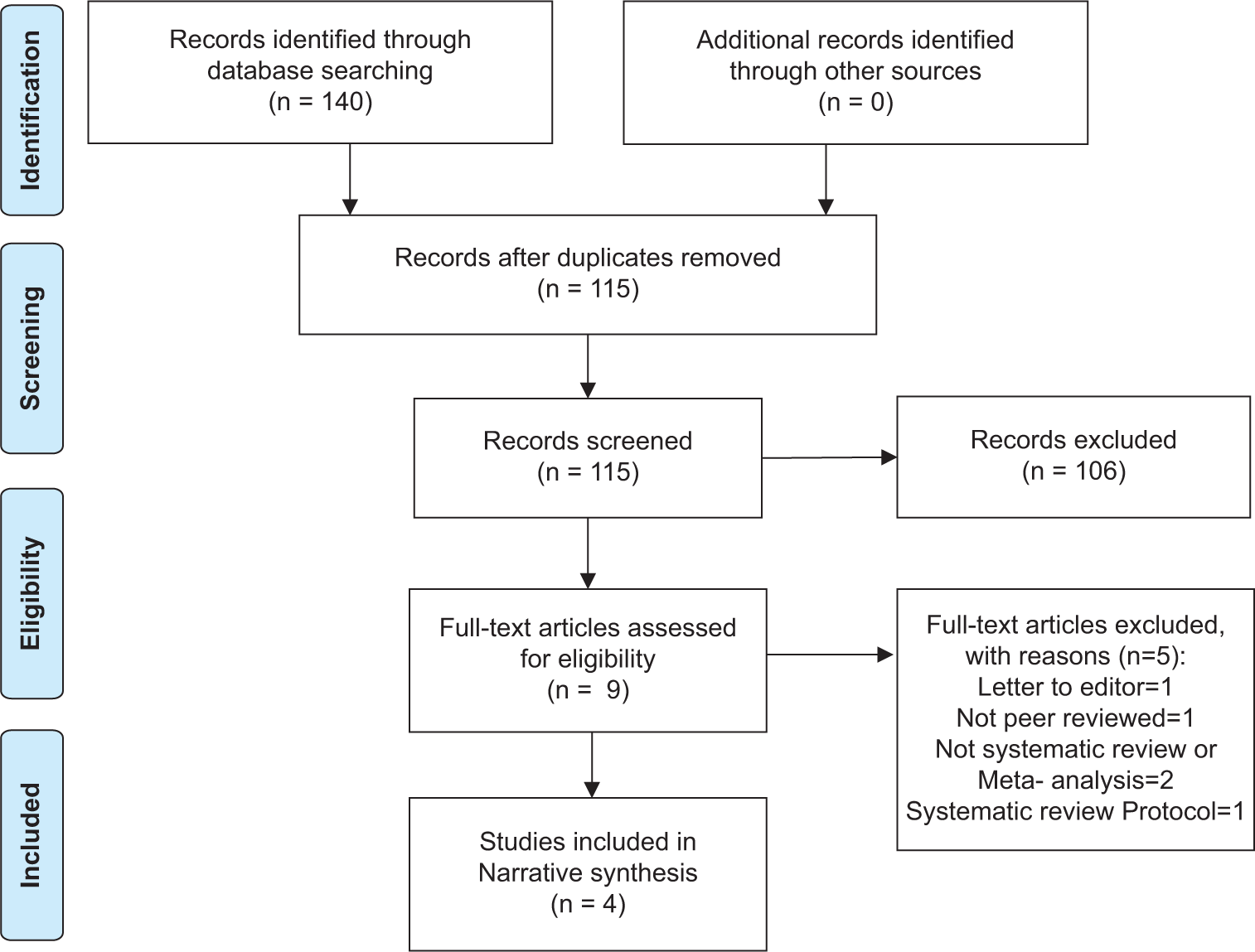
- The PRISMA flow diagram for the literature search.
Study characteristics and quality assessment
The AMSTAR quality assessment tool was used to assess the methodological quality of the included studies. The four studies included in this review received the following AMSTAR summary quality scores: 5, 6, 7, and 9 out of a possible 11 points (range 5–9, mean 6.75), with only one study[7] scoring a 9. Three of the included studies have a methodological quality rating of medium (range 5–8), while one of them[7] has a methodological quality rating of high.[9]
All studies included in this umbrella review were published in 2020. Two of the four studies included in this review (50%) were conducted in China,[3,5] one in Italy,[6] and one in Iran.[2] Primary studies were conducted in China (12 studies, 48%), the USA (7 studies, 28%), Europe (5 studies, 20%), and Turkey (1 study, 4%), involving a total of 1,197,507 participants, including 22921 COVID-19 infected (cases) and 1174584 COVID-19 uninfected (control) participants. Table 1 summarizes key data from the included studies.
| First author (year) | Golinelli et al. (2020) | Wu et al. (2020) | Pourali et al. (2020) | Liu et al. (2021) |
|---|---|---|---|---|
| Place (country) of primary studies | USA=3 China=2 Europe=1 Turkey=1 |
USA=1 China=3 |
USA=1 China=3 |
USA=2 Europe=4 China=4 |
| Total sample size | Cases=7503 Controls=962160 |
Cases=2855 Controls=31651 |
Cases=3180 Controls=135940 |
Cases=9383 Controls=44835 |
| Number of primary studies (analyzed) | 7 (7) | 5 (4) | 4 (4) | 17 (10) |
| Data sets | Cases mostly from hospitals Controls from: Blood donors=3 studies Normal population=1 study Non-COVID-19 patients=2 studies Individuals recorded in electronic health recorded system=1 study |
Cases mostly from hospitals Controls from: not reported |
Cases from Hospitals Controls from: not reported |
Not reported |
| Gender | Not reported | Only 2 primary studies reported gender (male=164, female=944) | Not reported | 6 primary studies reported gender Male=56.4% Female=43.6% |
| Age (case/control) | Not reported | Not reported | Not reported | Majority were adults 13–80-years-old |
| Race | Not reported | Not reported | Not reported | Asians: Case=11436 Controls=26172 Caucasians: Case=6326 Controls=10284 |
| Test | *PCR | Not reported | **RT-PCR using nasal and pharyngeal swab specimens | PCR or Clinical diagnostic criteria including epidemic history or clinical symptoms |
| Subgroup analysis | Performed: Based on the type of control population and country | Not performed | Not performed | Performed: based on pre-set variable (country, race, and study design) |
Association between ABO blood groups and COVID-19 infection
Three of four (75%) studies included in this umbrella review found a significant positive correlation between blood group A and the risk of contracting COVID-19. The odds ratio (OR) for this correlation varied between 1.16 (95% confidence interval [CI]: 1.02–1.33) and 1.33 (95% CI: 1.14–1.56) across the included studies.
According to one study,[5] participants with blood group A had a significantly increased risk of COVID-19 infection compared to non-A blood group participants (OR 1.33 95% CI: 1.14–1.56), while individuals with blood group B had a slightly increased risk of COVID-19 infection compared to non-B blood group participants (OR 1.06 95% CI: 1.00–1.13). Moreover, four of the ten studies included in the quantitative synthesis that demonstrated an association between the Rh blood group and COVID-19 infection indicated that the risk of COVID-19 infection was significantly associated with the Rh-positive blood group (OR 1.22 95% CI: 0.99–1.50).
Each of the four studies included in this umbrella review (100%) demonstrated a significant negative correlation between blood group O and COVID-19 infection. The ORs for this correlation ranged between 0.699 (95% CI: 0.66–0.88) and 0.77. (95% CI: 0.67–0.88).
Concerning COVID-19 severity, one study[3] discovered that the odds of COVID-19 severity were higher in individuals with blood group AB (OR 2.424 95% CI: 0.934–6.294) and lower in individuals with blood group O (OR 0.748 95% CI: 0.556–1.007).
Concerning mortality, three of the four included studies found no significant association between the ABO blood group and mortality outcome in COVID-19 patients; one study[4] discovered that blood group A was associated with a significantly increased risk of COVID-19 mortality, and one study[3] discovered that blood group AB was associated with an increased risk of death.
One study (25%) lacked reported data sets, while the remaining 3 (75%) reported that most COVID-19 cases occurred in the hospital.
Concerning the design of primary studies, 17 (68%) of the 21 primary studies that reported the design used a case-control design, 3 (12%) utilized a cohort design, and 1 (4%) employed a cross-sectional design.
Concerning the quality assessment of the primary studies using the Newcastle-Ottawa Scale, the majority (68%) were rated as medium (scored 4–6), while the remainder (32%) were rated as high (scored seven and over).
In terms of the overall conclusion, three of the four studies included concluded that blood group A may be a risk factor for COVID-19 infection, one study[5] concluded that blood groups A and B may be risk factors for COVID-19 infection, and all four studies included concluded that blood group O may be a protective factor. Furthermore, the risk of infection with COVID-19 was significantly associated with the Rh-positive blood group.
Three of the four studies included in this umbrella review used the random-effects model, and one study[3] analyzed the data using both random and fixed-effect models. One study[6] used subgroup analysis to determine the source of heterogeneity based on the type of control population and concluded that the type of control population had no significant effect on the observed heterogeneity. They did, however, discover a greater degree of homogeneity among studies that used the general population (I2 = 34.4%) compared to the others.
Each of the studies included, demonstrated a high degree of heterogeneity.
DISCUSSION
To the best of the authors’ knowledge, this is the first umbrella review examining the relationship between ABO blood groups and susceptibility to COVID-19 infection.
All of the studies included in this umbrella review (100%) suggested that blood group O may be associated with a reduced risk of susceptibility to COVID-19 infection. However, only 75% of the studies included in this study suggested that blood group A may be associated with an increased risk of COVID-19 infection. In other words, all of the studies included in this umbrella review concluded that blood type O might act as a protective factor against COVID-19 infection, while the majority concluded that blood type A might act as a risk factor.
This finding is consistent with some previous research on the association between ABO blood groups and SARSCoV-2 (COVID-19) infection.[8,13-16] This finding, however, contradicts recent meta-analytic studies on the association between ABO blood groups and SARS-CoV-2 (COVID-19) infection.
In addition, this umbrella review revealed that the findings from the included studies on the association between ABO blood groups and COVID-19 mortality outcomes were more inconsistent and mixed.
Moreover, the current umbrella review uncovered significant heterogeneity across all included studies, which could account for inconsistent, discordant, or mixed findings. Between-study differences, including methodological differences, such as differences in the treatment, the treated population, the study design, the study setting, the variations in ABO blood group frequencies between populations with varying geographical origins and ancestries, or the data analysis method, may contribute to such heterogeneity. In addition, methodological issues such as flaws in primary study design and participant differences[3,15,17-20] such as sex, age, ethnic origin, lifestyle, beliefs, smoking, and comorbidity with chronic diseases may contribute to the varied results.
For instance, when only data on critically ill patients are analyzed, critical risk factors such as sex, age, obesity, cardiovascular, and underlying metabolic diseases are overlooked. The ABO polymorphism has been linked to several of these COVID-19 risk factors.[15] The current umbrella review revealed that most studies included in this (75%) reported that the majority of COVID-19 cases occurred in hospitals.
Similarly, the study’s design was criticized for relying heavily on blood donors as controls. Using faulty controls is a well-known source of erroneous conclusions, and blood donors are generally favored for blood group O.[3,21] Two of the four studies reported that some primary studies used blood donors as controls, while the remaining studies did not report this information.
Furthermore, in many small meta-analyses, we may not accurately estimate heterogeneity.[19,22,23]
Moreover, according to Pei et al.[24], heterogeneity tests were frequently underpowered. As a result, the degree of heterogeneity may be greater than what is observed. Heterogeneity between studies may introduce false-positive results.
A long-standing concern about the validity of epidemiological findings has been the possibility of bias due to uncontrolled confounding. Even when known risk factors are controlled for, residual confounding may occur due to measurement error or unmeasured or unknown risk factors. Although residual confounding is challenging to eliminate in observational studies, the amount of information that this unknown confounding can explain is limited.[25] In addition, according to Dekkers et al.,[26] confounding is a significant threat to the validity of observational studies. Confounding occurs when comparison groups differ in terms of their risk of developing an adverse outcome other than the exposure(s) of interest due to a common cause of exposure and outcome. If the included studies have a low risk of bias and the observed heterogeneity is small, researchers may conclude that the primary findings provide reasonably valid estimates.[27]
The researchers emphasized the importance of investigating the reasons or sources of heterogeneity in meta-analyses, particularly those involving observational studies.[27-29]
Despite significant heterogeneity across studies, only 50% of the studies included in this umbrella review used subgroup analysis to investigate the sources of heterogeneity. Only 25% of the studies included in this umbrella review provided information about the participants’ age, gender, and race and investigated the association between Rh-type COVID-19 infection.
Although the mechanisms underlying the association between ABO blood groups and risk of COVID-19 infection and severity are not fully understood, several hypotheses regarding mechanisms linking Non-O blood group type increased susceptibility and severity compared to O blood group type can be advanced:
O blood group has anti-A antibodies,[5,16] which can target ACE2 and prevent cellular entry of SARS-COV-2, leading to COVID-19 infection.
The non-O blood group has higher furin (a cellular enzyme pertaining to the category of proprotein convertases) levels, which allow more activation of COVID-19 infection.
Non-O blood group, especially A group have a higher body mass index.
The hypercoagulable state is more seen in the non-O blood group.
Upregulated inflammation autoimmunity is more prevalent in the non-O blood group.[30-32]
Non-O blood group intestinal microbiota (Gut microbiota) are directed toward actinobacteria (pro-inflammatory).[33]
We added the diathesis-stress model, also known as the vulnerability-stress model, which is a psychological theory of psychopathology to explain our results regarding the association between ABO blood groups and the risk of COVID-19 infection and severity; however, further investigations are needed to test this model not only on psychopathology but also on various infectious and other noninfectious diseases. In other words, this model is applicable to all health conditions and problems.
The diathesis-stress model argues that certain pathological states or diseases emerge from the combination or interaction of predisposition (diathesis) with stressful events (stress). This model specifies that neither the diathesis nor stress alone is sufficient to produce the disorder. Instead, stress activates the diathesis, which then leads to the disorder. More broadly, the diathesis-stress models are similar to the idea of risk factors for stress-related diseases.[34] The stress-diathesis model recognizes that people have their unique diathesis (vulnerabilities and protections) to the experience of trauma, positioning these as pre-and-post-trauma experiences in a bio-psycho-socio-spiritual context.[35]
The biopsychosocial model was first conceptualized by Engle in 1977, suggesting that to understand a person’s medical condition, it is not simply the biological factors to consider but also the psychological and social factors.[36]
Biological factors such as genetic vulnerability, physical health, and comorbidities. Psychological factors such as perceived stress, psychological distress, psychological health, self-esteem, coping skills, cognitions, emotions, behaviors, lifestyle and psychological resilience, and social factors such as social networks, social support, and socioeconomic status. The interaction of biopsychosocial factors may lead to a specific health condition. The COVID-19 pandemic is a traumatic or severe stressful situation, which may lead to both adverse psychosocial consequences and COVID-19 infection, severity, and mortality due to impaired immune system through feedback loop in vulnerable people with non-O blood group. The interaction of psychological and biological resiliency may lead to “bio-psycho-neuro-immunological resiliency.” We believe that the diathesis-stress model may be potentially most helpful in explaining all human health conditions and problems, including COVID-19 and long COVID or post-COVID-19 conditions, infectious and non-infectious diseases. The COVID-19 pandemic is a traumatic or severe stressful situation, which may lead to both adverse psychological consequences and COVID-19 infection, severity, and mortality due to impairment and dysregulation of the immune system through feedback loop in vulnerable people with non-o blood group.
The interaction of psychological and biological resiliency may lead to “bio-psycho-neuro-immunological resiliency”.
The diathesis-stress model may be potentially most helpful in explaining all human health conditions and problems, including COVID-19 and Long COVID or Post-COVID-19 conditions, infectious and non—infectious diseases.
Because based on a biopsychological viewpoint as a holistic or systemic approach, there are no purely medical or biological health conditions in human being.
Although key stress hormones such as norepinephrine and glucocorticoids such as cortisol have been implicated in these processes, a direct link between the brain cells that coordinate the neuroendocrine stress response has remained elusive.[37] Bains and Sharkey[37] showed that in comparison to age-and-sex-matched controls, exposed to SARS-CoV-2 stressed mice had higher viral titers. These effects were also dependent on corticosterone. Furthermore, this attenuation of the response to the virus is not specific to SARS-CoV-2, as stress also increases viral titers after exposure to the influenza virus. Considerable data suggest that early-life and chronic stress, as well as acute stress, dysregulate both innate and adaptive immune systems by altering the balance of cytokines toward an inflammatory milieu. Resilient individuals have different innate and adaptive immunophenotypes from that of stress-sensitive individuals, but there remains a paucity of studies that directly assess the role of brain circuitry involved in these differences. The main lesson is that stress during the early phase of virus exposure impairs host adaptive immunity against infections.
Researchers from the University of Texas Health Science Center at San Antonio, working with collaborators in five countries, revealed that the capacity to resist or recover from infections and other sources of inflammatory stress, called “immune resilience,” differs widely among individuals. They also found that immune resilience is not age-dependent. Immune resilience is the capacity to maintain good immune function, called “immunocompetence,” and minimize inflammation while experiencing inflammatory stressors. They also showed that individuals with optimal levels of immune resilience were more likely to live longer, resist HIV and influenza infections, resist recurrence of skin cancer after kidney transplant, and survive COVID-19 infection and sepsis. However, the concept of immune resilience captures levels of immunocompetence and inflammation together.[38]
Abegaz[39], in a review, found that higher incidence of various types of cancers in the stomach, ovaries, salivary glands, cervix, uterus, and colon/rectal and thromboembolic diseases were more common in blood type A people than in O type people and concluded that it is now clear that ABO blood types are not the exact cause of diseases, but they can be susceptible and surrender to disease and health problems. In general, non-O blood types are more susceptible to disease than O.
In general, the evidence mentioned above appears to be in favor of the diathesis-stress model.
The following are some of the potential limitations of this umbrella review: First, due to the small sample size, the findings were limited. Second, this study’s omission of gray literature is another limitation.
However, this umbrella review’s strengths include searching multiple databases and limiting itself to peer-reviewed articles.
CONCLUSION
This umbrella review indicates that blood group A may be a risk factor for COVID-19 infection, whereas blood group O may be a protective factor. However, most of the studies included in this umbrella review reported significant heterogeneity across primary studies, limiting the findings. In addition, the mechanisms of the association between ABO blood groups and the risk of COVID-19 infection are not fully understood. Therefore, additional methodologically rigorous and experimental research, as well as prospective cohorts, are warranted regarding ABO blood group and COVID-19 infection, severity, and demise.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Relationship between the ABO blood group and the coronavirus disease 2019(COVID-19) susceptibility. Clin Infect Dis. 2021;73:328-31.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between blood group and risk of infection and death in COVID-19: A live meta-analysis. New Microbes and New Infect. 2020;37:100743.
- [CrossRef] [PubMed] [Google Scholar]
- Association between ABO blood groups and COVID-19 infection, severity and demise: A systematic review and meta-analysis. Infect Genet Evol. 2020;84:104485.
- [CrossRef] [PubMed] [Google Scholar]
- WHO coronavirus (COVID-19) dashboard. Available from: https://covid19.who.int [Last accessed on 2021 Aug 20]
- [Google Scholar]
- The impact of ABO blood group on COVID-19 infection risk and mortality: A systematic review and meta-analysis. Blood Rev. 2021;48:100785.
- [CrossRef] [PubMed] [Google Scholar]
- The association between ABO blood group and SARS-COV-2 infection: A meta-analysis. PLoS One. 2020;15:E0239508.
- [CrossRef] [PubMed] [Google Scholar]
- Smoking increases the risk of COVID-19 infection: A narrative review. Iran J Public Health. 2021;50:431-7.
- [CrossRef] [Google Scholar]
- Association between ABO blood types and coronavirus disease 2019(COVID-19), genetic associations, and underlying molecular mechanisms: A literature review of 23 studies. Ann Hematol. 2021;100:1123-32.
- [CrossRef] [PubMed] [Google Scholar]
- Preferred reporting items for systematic reviews and meta-analyses. The PRISMA statement. PLoS Med. 2009;6:e1000097.
- [CrossRef] [PubMed] [Google Scholar]
- Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10.
- [CrossRef] [PubMed] [Google Scholar]
- An AMSTAR assessment of the methodological quality of systematic reviews of oral healthcare interventions published in the Journal of Applied Oral Science (JAOS) J Appl Oral Sci. 2011;19:440-7.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- [CrossRef] [PubMed] [Google Scholar]
- ABO blood group and COVID-19: A review on behalf the ISBT COVID-19 working group. Vox Sang. 2021;116:849-61.
- [CrossRef] [PubMed] [Google Scholar]
- Association between ABO blood groups and susceptibility to COVID-19: Profile of age and gender in Iraqi patients. Egypt J Med Hum Genet. 2021;21:76.
- [CrossRef] [PubMed] [Google Scholar]
- ABO blood types and COVID-19: Spurious, anecdotal, or truly important relationships? A reasoned review of available data. Viruses. 2021;13:160.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of ABO blood grouping on covid-19 vulnerability and seriousness: A retrospective cross-sectional controlled study among Arab community. Int J Environ Res Public Health. 2021;18:276.
- [CrossRef] [PubMed] [Google Scholar]
- An overview of retrospective case-control studies investigating the relationship between prone sleeping position and SIDS. J Paediatr Child Health. 1991;27:334-9.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2020;21:1525-37.
- [CrossRef] [PubMed] [Google Scholar]
- The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med Methodol. 2015;15:35.
- [CrossRef] [PubMed] [Google Scholar]
- Smoking and risk of negative outcomes among COVID-19 patients: A systematic review and meta-analysis. Tob Induc Dis. 2021;19:9.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19: Risk of infection is high, independently of ABO blood group. Haematologica. 2020;105:2706-8.
- [CrossRef] [PubMed] [Google Scholar]
- Bias caused by sampling error in meta-analysis with small sample sizes. PLoS One. 2018;13:e0204056.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: A nationwide study. Eur J Epidemiol. 2021;36:287-98.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring the major sources and extent of heterogeneity in a genome-wide association meta-analysis. Ann Hum Genet. 2016;80:113-22.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749-54.
- [CrossRef] [PubMed] [Google Scholar]
- COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019;16:e1002742.
- [CrossRef] [PubMed] [Google Scholar]
- Sources of heterogeneity in the meta-analysis of observational studies: The examples of SIDS and sleeping position. J Clin Epidemiol. 2001;54:440-7.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges in meta-analyses with observational studies. Evid Based Ment Health. 2020;23:83-7.
- [CrossRef] [PubMed] [Google Scholar]
- Why sources of heterogeneity in meta-analysis should be investigated? BMJ. 1994;309:1351-5.
- [CrossRef] [PubMed] [Google Scholar]
- ABO blood group is associated with COVID-19 susceptibility: A systematic review and meta-analysis. Iberoam J Med. 2021;3:71-84.
- [CrossRef] [Google Scholar]
- Systematic review and meta-analysis of the susceptibility of ABO blood group to COVID-19 infection. Transfus Apher Sci. 2021;60:103169.
- [CrossRef] [PubMed] [Google Scholar]
- ABO blood group and COVID-19: An updated systematic review and meta-analysis. Blood Transfus. 2021;19:317-26.
- [Google Scholar]
- The potential use of ABO blood group system for risk stratification of COVID-19. Med Hypotheses. 2020;145:110343.
- [CrossRef] [PubMed] [Google Scholar]
- Diathesis-stress model In: Gellman MD, Turner JR, eds. Encyclopedia of behavioral medicine. New York: Springer; 2013.
- [Google Scholar]
- Integrating the gut microbiome and stress-diathesis to explore post-trauma recovery. An updated model. Pathogens. 2022;11:716.
- [CrossRef] [PubMed] [Google Scholar]
- The need for a new model: A challenge for biomedicine. Science. 1977;196:129-36.
- [CrossRef] [PubMed] [Google Scholar]
- Stress and immunity-the circuit makes the difference. Nat Immunol. 2022;23:1137-9.
- [CrossRef] [PubMed] [Google Scholar]
- Immune resilience despite inflammatory stress promote longevity and favorable health outcome including resistance to infection. Nat Commun. 2023;14:3286.
- [CrossRef] [PubMed] [Google Scholar]
- Human ABO blood groups and their associations with different diseases. Biomed Res Int. 2021;2021:6629060.
- [CrossRef] [PubMed] [Google Scholar]







