Translate this page into:
Azathioprine-induced severe pancytopenia: A serious complication in a patient with normal TPMT activity
*Corresponding author: Subhajit Hajra, Department of Pathology and Laboratory Medicine, All India Institute of Medical Sciences -Rishikesh, Uttarakhand, India. subhajithajra@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hajra S, Bera H. Azathioprine-induced severe pancytopenia: A serious complication in a patient with normal TPMT activity. J Hematol Allied Sci 2022;2:55-8.
Abstract
Azathioprine is an immunomodulator commonly used in renal transplant recipients and to treat lupus erythematous, and inflammatory bowel disease, and as second-line therapy in cases of rheumatoid arthritis. Here, we present a case of non-segmental vitiligo treated with low-dose azathioprine developing life-threatening pancytopenia with febrile neutropenia and trephine biopsy-proven drug-induced myelosuppression. The patient needed broad-spectrum antibiotics and granulocyte colony-stimulating factor (G-CSF) support with intermittent transfusion of blood products to completely recover from myelosuppression. It took almost a month for the patient to completely recover from the cytotoxicity caused by azathioprine. Interestingly, the patient had normal thiopurine methyl transferase (TPMT) activity and wild-type TPMT allele. We tried to outline how these types of patients can be managed and to emphasize the need for continuous monitoring of blood counts in patients receiving azathioprine to prevent life-threatening cytopenia even with normal TPMT activity and wild-type TPMT allele.
Keywords
Azathioprine
Pancytopenia
Myelosuppression
Thiopurine methyl transferase
Drug-induced cytopenia
INTRODUCTION
Azathioprine is an immunomodulator or a steroid-sparing agent used in various autoimmune disorders and its role has been popularized in vitiligo as well.[1] Azathioprine is used for the treatment of cytopenia; however, anecdotal reports exist for myelosuppression caused by the same rare event. Our case was found to develop severe myelosuppression following 4 months of azathioprine therapy despite having normal thiopurine methyl transferase activity (TPMT) – a key metabolizing enzyme of azathioprine.
CASE REPORT
A 27-year-old female with non-segmental vitiligo was treated by a dermatologist with 50 mg/ day oral azathioprine and 5 g/day oral betamethasone on 2 consecutive days per week. She presented with fever, oral ulcer, dyspnea on exertion, and severe pallor 4 months later. No baseline autoimmune workup was done as the patient had no other clinical features apart from vitiligo.
A complete hemogram revealed hemoglobin (Hb) of 3.5 gm/dl, total leukocyte count (TLC) of 2,300/cu.mm, absolute neutrophil count (ANC) of 164/cu.mm, and platelet count of 40,000/cu.mm. The liver function test showed mild elevation of aspartate aminotransferase (39U/L) and Alanine aminotransferase (72U/L). Azathioprine and steroid were stopped. She was transfused with 2 units of packed red blood cells immediately. A bone marrow aspiration and biopsy were performed. Simultaneously, she was put on 300 mcg/12 hourly (body weight – 54 kg) granulocyte colony-stimulating factor (G-CSF) and broad-spectrum antibiotic with piperacillin and tazobactam and amikacin. Blood culture after 48 h showed no growth. Bone marrow aspiration and biopsy were done to investigate the cause of pancytopenia. Aspirate smears are markedly hemodiluted with peripheral blood; however, trephine biopsy was markedly hypocellular with overall cellularity of <10% with interstitial prominence of small mature lymphocytes and plasma cells [Figure 2]. TLC started improving from the 3rd day after initiating G-CSF with falling platelet count and Hb. Packed red cell concentrates and platelet concentrates were given to keep Hb >8.0 g/dl and platelet count >20,000/cu.mm, respectively. A further blood culture after 5 days showed growth of coagulase-negative staphylococcus epidermidis. Antibiotics were changed to meropenem and teicoplanin. The fever subsided after 4 days and the antibiotics were continued for 15 days. G-CSF dose was reduced to 300 mcg/day after ANC of more than 1000/ cu.mm was achieved after 10 days of initiating G-CSF. Finally, G-CSF was stopped after 17 days. The patient was discharged after 20 days after admission with a TLC of 2,460/cu.mm and ANC of 615/cu. mm with Hb of 8.3 gm/dl and platelet count of 46,000/cu.mm [Figure 1 and Table 1].
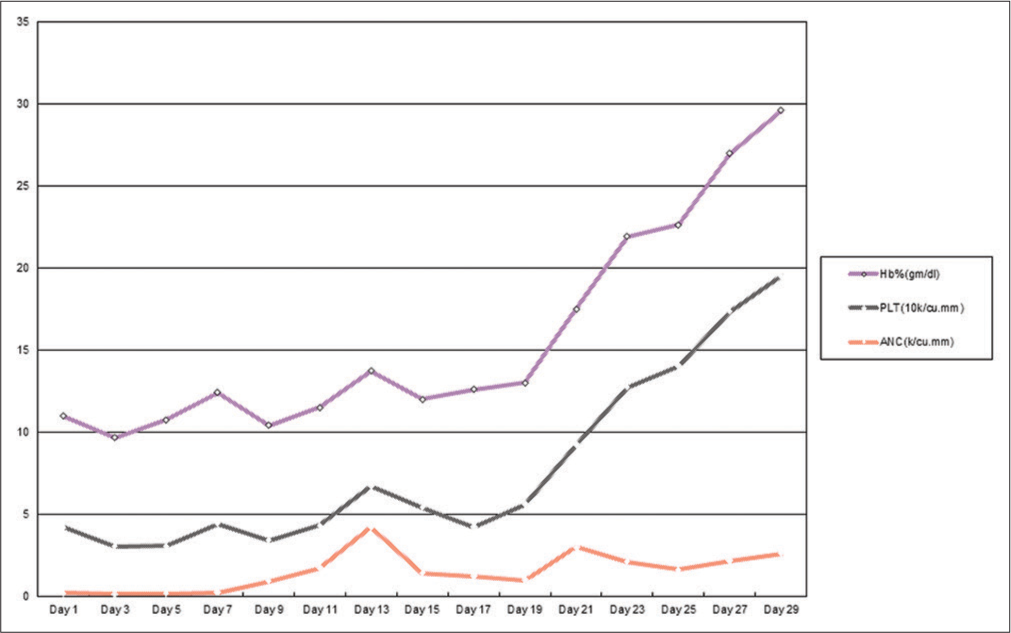
- The clinical course of different hematological parameters over 1 month.

- Low-power view (H&E stain; 100×) of bone marrow trephine biopsy shows hypocellular marrow with respect to age and sex (a). The high-power (H&E stain; 400×) view shows the interstitial prominence of lymphocytes and plasma cells (b).
| Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | Day 15 | Day 17 | Day 19 | Day 21 | Day 23 | Day 25 | Day 27 | Day 29 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANC (k/cu.mm) | 0.168 | 0.135 | 0.15 | 0.2 | 0.9 | 1.7 | 4.2 | 1.4 | 1.2 | 0.98 | 3 | 2.1 | 1.61 | 2.15 | 2.59 |
| PLT (10k/cu.mm) | 4 | 2.9 | 2.9 | 4.2 | 2.5 | 2.6 | 2.5 | 4 | 3 | 4.6 | 6.2 | 10.6 | 12.4 | 15.2 | 16.9 |
| Hb% (g/dl) | 6.8 | 6.6 | 7.7 | 8 | 7 | 7.2 | 7 | 6.6 | 8.4 | 7.4 | 8.3 | 9.2 | 8.6 | 9.6 | 10.1 |
ANC: Absolute neutrophil count, PLT: Platelet
The patient was referred to a dermatologist for the treatment of vitiligo and advised to use treatment regimens that do not include azathioprine.
During the follow-up for next 4 months, the ANC, platelet count, and Hb level were found to be sustaining at a normal range without any further intervention.
TPMT genotyping was done 6 weeks after she was discharged and, revealed a wild genotype with normal TPMT activity.
DISCUSSION
Azathioprine is rapidly and non-enzymatically converted to 6-mercaptopurine. Then, 6-mercaptopurine is converted to thiopurine nucleotide analogues, initially by the enzyme hypoxanthine-guanine phosphoribosyltransferase and then by multienzyme steps. These analogs are incorporated into DNA. This mechanism is responsible for the cytotoxic action of azathioprine.[2]
Azathioprine is metabolized by two different pathways, predominantly by the TPMT and xanthine oxidase pathways. Xanthine oxidase is absent in hematopoietic tissue and it does not show any genetic polymorphism, whereas TPMT shows genetic variability and several forms of variant alleles in the general population with very low-level/complete deficiency of the enzyme are seen in every 1 in 300 in the western population.[3]
Patients on azathioprine are to be closely monitored regarding their hematological parameters at regular intervals to identify impending crises arising from myelosuppression.
Bone marrow suppression due to azathioprine was reported to be 14–35% of the patients.[4] An Indian study has found the chances of developing bone marrow suppression in patients on azathioprine to be 14.3%.[5]
Mcgrath et al. described three phases of bone marrow suppression in patients treated with azathioprine – the stage of megaloblastic erythropoiesis, the stage of defective myelopoiesis, and the stage of toxic myelopathy.[6]
Leipold et al., showed that the patients with mutated TPMT genotype are jeopardized by severe pancytopenia during azathioprine therapy.
Ari et al. and Hadda et al. also advocated screening for TPMT activity before commencing azathioprine therapy to identify patients with an increased risk of developing severe myelosuppression.[7-9]
As suggested by the previous studies, a study from China showed that TPMT polymorphism is relatively rare in the Asian population. Moreover, this study also identified two other genetic polymorphisms. The nucleoside diphosphate-linked moiety X motif 15 (NUDT15 [415C>T] polymorphism) had a statistically significant correlation between myelosuppression and mutated genotype with homozygotes being more prone to develop severe myelosuppression compared to heterozygotes for this polymorphism. This study also included a patient developing severe myelosuppression despite having a wild-type genotype for all three genes implicated.[10]
As azathioprine is a common immunomodulator and commonly used in many autoimmune diseases, the rare occurrence of life-threatening cytopenia in these patients remains a possibility and should be treated aggressively with broad-spectrum antibiotics and growth factor support. Hb and platelet count should also be maintained at the desired level with transfusion of blood products as and when required. Frequent monitoring of blood counts is of immense help to determine treatment decisions. Moreover, as is seen in this case, it may take a substantial time before the counts start recovering. The crux of our case is despite having normal TPMT activity, our patient life was endangered by severe cytopenia arising from Grade B myelosuppression,[11] which eventually required long-standing G-CSF therapy for almost 3 weeks along with broad-spectrum antibiotic prophylaxis and stringent barrier nursing.
CONCLUSION
Although most of the reported cases of azathioprine-induced myelosuppression were associated with deficient or absent TPMT activity, our experience showed that severe myelosuppression may occur even in patients with normal TPMT activity. As recent studies suggest the role of NUDT15 polymorphism and myelosuppression, these patients may be considered to be tested for this polymorphism also before starting therapy. Moreover, we also recommend that close and frequent monitoring of hematological parameters at least fortnightly in the early period of long-term azathioprine therapy is the best possible and cost-effective predictor of impending bone marrow suppression compared to the huge cost of long-term G-CSF and broad-spectrum antibiotic therapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Azathioprine in dermatological practice. An overview with special emphasis on its use in non-bullous inflammatory dermatoses. Adv Exp Med Biol. 1999;455:343-8.
- [CrossRef] [PubMed] [Google Scholar]
- Azathioprine: Current status and future considerations. Int J Dermatol. 2003;42:335-41.
- [CrossRef] [PubMed] [Google Scholar]
- The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics. 1999;9:37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Leucopenia and azathioprine management in renal homotransplantation. Surgery. 1972;71:598-604.
- [Google Scholar]
- Azathioprine-induced Bone Marrow Suppression. Abstract 4D 0108. In: Asia Pacific Congress of Nephrology. 1992.
- [Google Scholar]
- Erythroid toxicity of azathioprine. Macrocytosis and selective marrow hypoplasis. Q J Med. 1975;44:57-63.
- [Google Scholar]
- Azathioprine-induced severe pancytopenia due to a homozygous two-point mutation of the thiopurine methyltransferase gene in a patient with juvenile HLA-B27-associated spondylarthritis. Arthritis Rheum. 1999;40:1869-98.
- [CrossRef] [PubMed] [Google Scholar]
- Azathioprine-induced myelosuppression due to thiopurine methyltransferase deficiency in a patient with autoimmune hepatitis. J Hepatol. 1995;23:351-4.
- [CrossRef] [Google Scholar]
- Azathioprine induced pancytopenia: A serious complication. J Postgrad Med. 2009;55:139-40.
- [CrossRef] [PubMed] [Google Scholar]
- Association between genetic polymorphisms of metabolic enzymes and azathioprine-induced myelosuppression in 1,419 Chinese patients: A retrospective study. Front Pharmacol. 2021;12:672769.
- [CrossRef] [PubMed] [Google Scholar]
- Myelosuppression grading of chemotherapies for hematologic malignancies to facilitate communication between medical and dental staff: Lessons from two cases experienced odontogenic septicemia. BMC Oral Health. 2013;13:41.
- [CrossRef] [PubMed] [Google Scholar]






