Translate this page into:
CAR-T therapy and allogeneic stem cell transplantation for refractory acute myeloid leukemia: A comparative study
*Corresponding author: Nasser Ghaly Yousif, Department of Medicine, Medical College, Al Muthanna University, Samawa, Al Muthanna, Iraq. yousif_ghaly@mu.edu.iq
-
Received: ,
Accepted: ,
How to cite this article: Yousif NG, Nöth UA, Al-Amran FG. CAR-T therapy and allogeneic stem cell transplantation for refractory acute myeloid leukemia: A comparative study. J Hematol Allied Sci. 2025;5:11-7. doi: 10.25259/JHAS_44_2024
Abstract
Children with chemo-refractory acute myeloid leukemia (AML) have a poor prognosis and a high frequency of relapsed and/or refractory AML, with a poor result of hematopoietic stem cell transplantation (HSCT). An external major histocompatibility antigens-independent antigen-binding domain, a transmembrane-linking domain, and an intracellular costimulatory T-cell signaling domain or numerous domains make up chimeric antigen receptor T lymphocytes (CARTs). By specifically targeting CD19, CART have proved effective in improving therapy results for B-lineage acute lymphoblastic leukemia. AML is defined as the absence of a myeloid counterpart to CD19, or a “expendable” antigen. Occasionally, AML will produce the T-cell antigen CD7, which anti-CD7 CAR-T-cells can target to destroy CD7-bearing T-cells. In conclusion; myeloid antigen-directed CART-cell therapy might cause remission in AML patients who are not responding to treatment and myeloid antigen-directed CAR-T therapy can be used as a bridge to allogeneic HSCT in r/r AML.
Keywords
Acute myeloid leukemia
Chimeric antigen receptor T-cells
Hematopoietic stem cell transplantation
INTRODUCTION
Acute myeloid leukemia (AML)
With a 5-year survival rate of only 30.5%, AML is one of the most aggressive hematological malignancies in adults and makes up just 1% of all new cancer cases in the US. AML patients have a dismal prognosis; the cure rate is about 5–15% in those over 60 and 35–40% in those under 60.[1] Most of the time, when the disease is first diagnosed, it responds well to high-dose induction chemotherapy; nevertheless, 10–40% of individuals are essentially resistant to induction chemotherapy. For intermediate- and high-risk AML patients, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is considered the only effective therapy currently available since it is anticipated to provide long-lasting complete remission (CR)[2] still, 50%.
A second hematopoietic stem cell transplantation (HSCT)
For the treatment of AML patients who relapse after a first HSCT, a second HSCT is an additional option. Unfortunately, there are considerable toxicities associated with a second HSCT, which makes few fit individuals suitable, particularly in the early post-transplantation phase.[3] Only a small percentage of patients (15%) who relapsed after their initial HSCT were able to receive a second transplant; response rates ranged from 41% to 56%, and the 2-year overall survival (OS) was between 15% and 42%. Comparably, only 369 patients (21%), in a trial conducted by Center for International Blood & Marrow Transplant Research (CIBMTR), were able to have a second HSCT; of these, 49% had myeloablative conditioning regimens, and 30% had decreased intensity/non-myeloablative regimens.[4] The second HSCT produced a stronger response, but only a small percentage of relapsed patients could afford it. The use of a reduced-intensity conditioning regimen has progressively increased over the past years, reaching 77% of patients in a recent large registry analysis.[5]
To produce a greater graft-versus-leukemia (GvL), there is currently insufficient evidence to recommend using a different donor for the second HSCT. The German transplantation group conducted retrospective registry research on 179 s HSCT (matched sibling donors; MSD, n = 75; matched unrelated donors; MUD, n = 104) using either the same or a new donor to explore the impact of donor change for acute leukemia relapsing after a first HSCT. Following a first HSCT from MSD versus MUD, the results of the second HSCT typically seemed better (2-year OS: 37% vs. 16%, respectively; hazard ratio (HR), 0.68; 95% confidence interval, 0.47 to 0.98; P = 0.042). It is interesting to note that choosing a different donor for the second HSCT did not considerably increase OS.[6]
There were no differences in response rates or survival between patients receiving a second HSCT from the same donors, a different matched donor, or a haploidentical donor in the context of haploidentical HSCT, according to a retrospective analysis involving 556 patients who relapsed after the first HSCT. At 2 years, leukemia-free survival was 23.5%, 23.7%, and 21.8%, respectively (P = 0.30); nevertheless, greater non-relapse mortality was expected by a haploidentical donor.[7,8] Overall, donor substitution did not hurt any of these investigations and is now a standard therapeutic procedure. Other researchers found that the percentage of AML patients who used a different donor increased significantly over time, from 31% in 2000–2004 to 80% in 2015–2018, in a sizable registry analysis of 8162 patients who relapsed after transplantation between 2000 and 2018. Furthermore, a higher OS rate of over 30% has been reported in both younger and older patients, including those who experienced an early recurrence. Extended disease control may be achieved in patients with high-performance status, full remission at the time of the second HSCT, and – most importantly – longer remission duration following the first HSCT, according to multivariate analysis in many trials.
CAR-T-cells
Three components usually make up chimeric antigen receptors (CARs): A transmembrane-linking domain, an intracellular costimulatory T-cell signaling domain or multiple domains, and an extracellular major histocompatibility antigens-independent antigen-binding domain that is typically derived from a single-chain variable fragment of a monoclonal antibody.[9] An extracellular spacer domain is also present in several CARs [Figure 1].
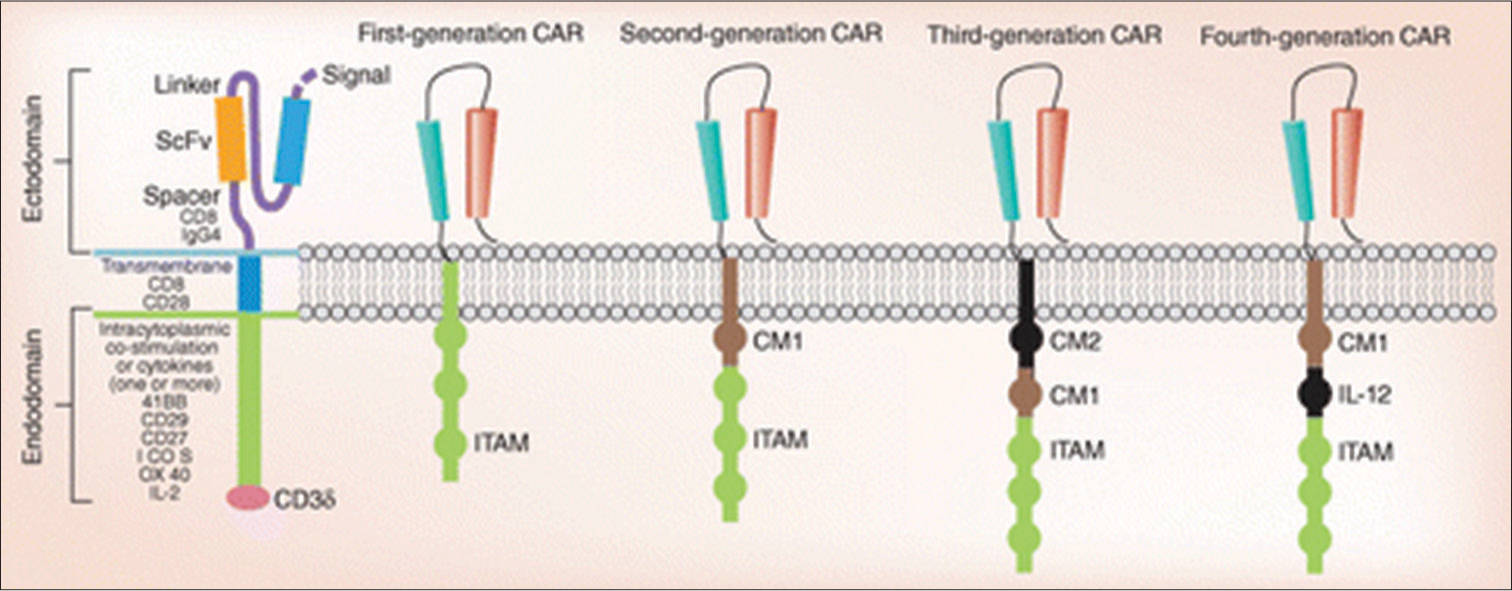
- Composition of a chimeric antigen receptor and the generations of chimeric antigen receptors. CAR: Chimeric antigen receptors, ITAM: Immunoreceptor tyrosine-based activation motifs, IL: Interleukin-2.
The components of a CAR consist of an extracellular single-chain variable fragment that is connected to a transmembrane domain (either CD8 or CD28) through a hinge (either CD8 or Immunoglobulin G 4), one or more intracellular costimulatory molecules (either Interleukin [IL]-12 or 41-BB, CD28, CD27, Inducible T-cell co-stimulator; ICOS, and/or OX40), or cytokines (31–3z). The various generations are distinguished by the immunoreceptor tyrosine based activation motif (ITAM) and several costimulatory molecules at the transmembrane region. A single-chain antibody (CD3ζ or FcεRIγ) linked the ITAM in the first generation. Based on the first generation, the second generation included a costimulatory molecule (CM1). In the third generation, there was an additional CM2. The fourth generation of CARs was produced by combining IL-12 with second-generation CARs.
Thus far, four generations of CAR-T-cells are shown Figure 1. The initial generation of CARs activated T-cells through the CD3η intracellular signaling domain, often known as the “first signal.” Nevertheless, these CARs exhibited limited activation, expansion, and in vivo antitumor effects, and did not release enough IL-2.[7] Then, by adding costimulatory molecules with intracellular costimulatory signaling domains, such as CD28, CD137 (4–1 BB), CD134 (OX40), CD27, ICOS, and CD244, the second generation of CARs was created. In comparison to the first generation, the second generation of CARs generated 20 times as much IL-2 and demonstrated sustained target cell death and growth in vivo.[1] By combining two or more costimulatory molecules with intracellular costimulatory signaling domains, the third generation of CARs was created. T-cell redirected for universal cytokine killing, or T cell redirected for universal cytokine-mediated killing (TRUCK) is the name given to the fourth generation of CARs. These cells are created by introducing a series of cytokines, such as IL-12, which can kill tumors by releasing cytokines locally to control the microenvironment and attract immune effector cells. Cancer cannot be treated using TRUCKS, but it can be used to treat metabolic problems, autoimmune illnesses, and viral infections.[7]
CAR molecules
CAR molecules are artificial chimeric receptors made up of the intracellular signaling domain of the T cell receptor (TCR) and an extracellular antigen-binding domain that is obtained from an antibody. Through T-cell modification, chimeric antigen receptor T lymphocytes (CART) treatment produces CARs that, in an human leukocyte antigen (HLA)- independent way, cytotoxically attack cancerous cells by recognizing a particular antigen expressed on their surface.[10] Leukapheresis is used to separate autologous T-cells from patient blood. These cells are then activated, genetically modified ex vivo to express the CAR on their surface, and grown in close-manufacturing bioreactors. These cells are usually cryopreserved once the cell product has been obtained and characterized. Before receiving CART infusion, patients often receive lymphodepletion chemotherapy to guarantee the engraftment of CAR-T-cells [Figure 1]. Finally, they are injected into the patient with the express purpose of killing cancer.
OVERVIEW OF CAT-T-CELL THERAPY FOR HEMATOLOGIC MALIGNANCIES AND ITS LIMITATIONS
By November 2023, nine CAR-T-cell products will have received global approval following the August 2017 introduction of the first CAR-T-cell product. Tisagenlecleucel (Kymriah), axicabtagene ciloleucel (Yescarta), brexucabtagene autoleucel (Tecartus), lisocabtagene maraleucel (Breyanzi), relmacabtagene autoleucel (Carteyva), and inaticabtagene autoleucel are among the CD19-targeted cell drugs among them, with the main indications for B-cell acute lymphoblastic leukemia (B-ALL), diffuse large B cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma, mantle cell lymphoma, and chronic lymphocytic leukemia (CLL).[11]
Idecabtagene vicleucel (Abecma), ciltacabtagene autoleucel (Carvykti), and equecabtagene autoleucel (Fucaso) are three B-cell maturation antigen (BCMA)-targeting cellular drugs, with multiple myeloma (MM) serving as the primary indication.[11] CAR-T-cell therapy has changed the way hematologic cancers are treated, and two drugs that target CD19 and BCMA have been very effective. The CR rates of CD19-targeted CAR-T in the treatment of R/R B-ALL were 62–86%, and the median event-free survival (EFS) was 3.1– 24 months.[12] The CR rates of R/R B-cell lymphoma and/or CLL were 28–68%, and the median EFS was 2.8–55 months.
For myeloid and lymphocyte hematologic malignancies, research progress has fallen behind that of B-cell hematologic malignancies due to the difficulties of choosing explicit targets and overseeing poisonous aftereffects. In intense myeloid leukemia, CD33, CD123, CD7, CD38, FLT3, Lewis Y, WT1, CLL1, NKG2D, and others are viewed as expected focuses of Vehicle T treatment, and related clinical preliminaries are in progress. It is important to note that CD123 and other antigens are expressed in healthy hematopoietic stem cells and progenitor cells in addition to leukemic blasts and stem cells. Due to this, targeting CD33 or CD123 with CAR-T cells will inevitably result in myeloablation, which can only be used as a bridging treatment for subsequent allo-HSCT. Although the Vehicle White blood cell treatment focusing on CD7 does not bring about myeloablation, some leukemic impacts do not communicate CD7 which might prompt a safe break. In addition, normal T-cells and NK cells express CD7, so the potential therapeutic cell-killing effect cannot be overlooked.[13] For hematologic malignancies including Lymphocytes, the improvement of Vehicle Immune system microorganism treatment is seriously difficult. While CART-cell fratricide and T-cell aplastic disorders need to be addressed, research on CAR-T-cell therapy for targets such as CD5, CD4, CD7, and CCR4 is still ongoing. After naturally selected CD7-targeted CAR-T-cell therapy, a clinical study on 20 patients with R/R T-ALL or T-cell lymphoblastic lymphoma revealed that the CR rate of minimal residual disease (MRD)-negative in the bone marrow was 95%, 18 patients developed grade 1–2 cytokine release syndrome (CRS), one patient developed grade 3 CRS, and two patients developed grade 1 neurotoxicity.[14] CAR-T-cell therapies for T-cell hematologic malignancies are still in the clinical trial stage, and clinical trials are needed to verify their long-term efficacy and safety. Even though Vehicle Lymphocyte treatments have accomplished critical viability in treating a few hematologic malignancies, there are still patients or illnesses that answer ineffectively to the treatment. Relapse occurred in 166 (44%) of the 376 children and young B-ALL patients who achieved CR after receiving CD19-targeted CAR-T treatment. Of the 376 patients who achieved CR, CD19-positive patients accounted for 50%, CD19-negative patients accounted for 41%, and lineage transition accounted for 7%. Different systems can add to the ineffectualness of Vehicle Lymphocytes, such as growth antigen avoidance, unusual growth apoptosis, Vehicle Lymphocyte fatigue, an immunosuppressive microenvironment, dealing with cancers and actuation problems, and Vehicle White blood cell initiated poisonousness responses.
CAR-T-CELLS ENGINEERED WITH NOVEL HYDROLASES/DRUG-LOADED NANOPARTICLES/DRUG-GATED SWITCHES
The execution of Vehicle White blood cell properties to aid atomic designated drug treatment is an extra thought for mix treatment. For instance, Tariq E et al. developed a Synthetic Enzyme-Armed KillER cell that, in accordance with the function of CAR-T-cells, secretes additional hydrolases. This cell is able to activate small molecule drug precursors in particular regions of the tumor and eminently maintain prodrug activation activity even after immune cells are exhausted.[15] These Vehicle Lymphocytes target and initiate little atom drugs with symmetrical action, joined with hostile to growth antecedent medications can improve the capacity to kill growths and stay compelling against antigen-negative cancer cells. While maintaining their activity, the CAR-T-cells were able to successfully transport drug-loaded nanoparticles into the tumor microenvironment (TME). CAR-T-cells that have been exhausted in the TME can be rescued and anti-tumor effects are enhanced when A2aR inhibitors are transferred together.[16] In addition, drug-gated switch CARs based on proteases and their inhibitors can effectively enhance CAR-T-cells’ safety and effectiveness. Specialists planned an ON-switch Vehicle consolidating NS3 cis-protease and cleavage site inclusions, as well as an OFF-switch Vehicle comprising a two-part parted polypeptide structure including NS3-restricting peptide and NS3 primary space, correspondingly.[17] When NS3 inhibitors are added, the former can mediate signaling blockage by competitive binding of NS3 structural domains with a peptide, while the latter can split the two-component linkage structure and restore CAR’s structural integrity by inhibiting the cleavage of CAR by NS3 protease. Both exchanging modes can accomplish explicit command over Vehicle T movement. In the interim, drug-flexible Vehicle stages permit Vehicle Lymphocytes to enter a calm state and keep away from persistent excitement by tonic signs, profiting from the inversion of White blood cell weariness and interceding uplifted enemy of growth movement. Improved therapeutic outcomes are being achieved by innovative combination modalities that broaden the range of applications for CAR-T-cells and molecularly targeted drugs.[18] Notwithstanding, the consolidated impacts of atomic designated medications and Vehicle White blood cell treatment are changed and multifaceted, and the current examination stays restricted. Studies are expected to additionally explain the atomic components by which blend treatments work, as well as bigger clinical preliminaries to analyze the viability and security of these treatments with monotherapies. At present, a few clinical preliminaries of microscopically designated drugs mixed with Vehicle Immune system microorganism treatment are in progress.[19]
CHIMERIC ANTIGEN RECEPTOR T-CELLS AND AML
On CAR-T-cells directed against myeloid antigens such as CD33, CD38, and CD123, as well as CD19, which is aberrantly expressed in AML with t(8;21);[20] there are currently reports that include patients who relapsed after HSCT. A new meta-examination including 57 patients revealed a pooled overall response rate (ORR) and CR of 65.2% and 49.5%, separately, while the term of reaction and operating system was exceptionally factor. The mixture of Vehicle Immune system microorganisms post-HSCT demonstrated protected across studies, yet viability stays humble, hence featuring the requirement for additional imminent preliminaries with a more noteworthy number of patients and longer development. Notably, the risk of graft reacts against the host (GvHD) resulting from T-cell alloreactivity is the primary concern associated with CART-cell therapy in the post-HSCT setting. Utilizing regulatory T-cells, TCR-deficient T-cells, and allogeneic virus-specific T-cells are three different strategies for mitigating GvHD. Besides, the inclusion of a self-destruction quality could address a procedure to oversee GvHD flares and serious poison levels by empowering quick removal of Vehicle Immune system microorganisms.[21] One more chance to alleviate GvHD in the post-transplantation setting is to produce Vehicle Lymphocytes from the beneficiary’s immune system microorganisms. Several case series have reported promising anti-leukemic efficacy with a low incidence of both acute and chronic GVDH, despite the possibility of lower reactivity. Other than decency, a promising endeavor to work on the remedial movement of Vehicle Immune system microorganisms included the upgrade of Lymphocyte homing into the bone marrow specialty by designing Vehicle Immune system microorganisms with overexpression of CXCR4, hence expanding the contact with remaining AML cells in preclinical models.[22] Focusing on the bone marrow’s dangerous microenvironment could address one more method for bypassing the obstruction instruments of AML. Dual CAR-T-cells that target both CD33 on myeloid leukemia cells and the mesenchymal stromal marker CD146 have been developed with this goal in mind, and their effectiveness against AML has improved. At last, epitope quality altering of contributor hematopoietic undifferentiated cells as of late showed up as a fruitful procedure to take apart on-track/on-growth action from on-track/off-cancer poisonousness of immunotherapies in preclinical models of AML backslid post-HSCT, conceivably addressing the reason for the improvement of a novel, proficient compound.[23]
Three distinct institutions simultaneously reported the successful clinical outcomes of CAR-T-cells: (1) the Public Malignant Growth Organization (National Cancer Institute; NCI) and (2) the Dedication Sloan-Kettering Malignant Growth Community (Memorial Sloan Kettering physicians; MSKCC) at the College of Pennsylvania (UPenn).[24] Patients were used to test many second-generation CART therapies that target the CD19 antigen. The NCI bunch treated a patient with cutting-edge follicular lymphoma, bringing about a fractional reaction and B-cell aplasia after Truck treatment. At MSKCC Emergency clinic, nine patients determined to have obstinate ongoing CLL or backslid B-ALL were treated with Truck treatment, showing both well-being and promising helpful potential. In the meantime, an anti-CD19 CART-treated refractory CLL patient’s initial complete response was reported by UPenn.[25] Intense AML is a heterogeneous neoplasm described by uncontrolled clonal extension of changed youthful hematopoietic forerunners, frequently connected with repetitive hereditary modifications. It has a median age of 68 years and an incidence of approximately 4.2 cases per 100,000 people, but it can occur at any age.[26] Chemotherapeutic agents, which typically consist of cytarabine and anthracyclines in the majority of cases, continue to be the treatment of choice for patients who are eligible for intensive treatment. Notwithstanding, new medications are logically being presented. For patients who are not qualified for concentrated therapies, novel dynamic low-power regimens have arisen and give significant antileukemic movement. These incorporate the blend of the bcl-2 inhibitor, venetoclax, with hypomethylating specialists or low-portion cytarabine, as well as the isocitrate dehydrogenases 1 (IDH1) inhibitor, ivosidenib, for AML cases holding onto an enacting change in this metabolic quality.[27] In any case, none of these remedial methodologies lead to finished leukemia destruction, and most patients will encounter a clinical movement after a few cycles. Due to its potential to eliminate residual leukemic cells through the GvL effect, allo-HSCT has been a pivotal procedure for non-favorable risk AML.[28] Backslid or stubborn (R/R) AML presents a successive testing situation, happening in 40-half of more youthful patients and, surprisingly, more much of the time in more established people. A standard treatment convention for these patients is as yet missing and endurance is poor, with a general endurance pace of 10% at 5 years.[29-30] Thus, there is an unmistakable requirement for novel restorative methodologies in this situation.[29] The remedial scene of AML has gone through ongoing change with the presentation of monoclonal antibodies (mAb, for example, CD33 gemtuzumab ozogamicin) and designated treatments such as the previously mentioned venetoclax, as well as IDH1/2 and FLT3 inhibitors. In addition, new approaches to immunotherapy are being investigated. For instance, the CD123 × CD3-targeting elotuzumab-engaging bispecific antibody’s efficacy is the subject of ongoing clinical trials.[31]
CONCLUSION
After allo-HSCT, patients with relapsed B-cell cancer benefit from allogeneic CAR-T-cells, which have few side effects and complications. As of late, coinfusion of haploidentical contributors determined CD19-Vehicle Immune system microorganisms and prepared fringe blood undeveloped cells following acceptance chemotherapy was acted in backslid and headstrong ALL. A third component can now be included in salvage regimens thanks to the recent addition of novel AML drugs to the arsenal. IDH1/2 inhibitors Ivosidenib and Enasidenib, as well as FLT3 inhibitors Sorafenib and Gilteritinib, can be combined with hypomethylating agents (HMA) and donor lymphocyte infusion (DLI) for patients with targetable mutations. Preventive intervention in the event of a molecular relapse is frequently linked to higher response rates and longer survival across studies. In this way, the requirement for close negligible remaining illness checking after HSCT and early mediation is fitting.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Advances in molecular targeted drugs in combination with CAR-T cell therapy for hematologic malignancies. Drug Resist Updat. 2024;74:101082.
- [CrossRef] [PubMed] [Google Scholar]
- CAR T-cells for T-cell malignancies: Challenges in distinguishing between therapeutic, normal, and neoplastic T-cells. Leukemia. 2018;32:2307-15.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcomes following CAR T cell therapy: What we know so far. Nat Rev Clin Oncol. 2024;20:359-71.
- [CrossRef] [PubMed] [Google Scholar]
- An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia. 2018;32:1970-83.
- [CrossRef] [PubMed] [Google Scholar]
- Allogeneic donor-derived myeloid antigen directed CAR-T cells-for relapsed/refractory acute myeloid leukemia in children after allogeneic hematopoietic stem cell transplantation: Report of three cases. Blood. 2022;140(Suppl 1):4600-1.
- [CrossRef] [Google Scholar]
- Outcomes with chimeric antigen receptor t-cell therapy in relapsed or refractory acute myeloid leukemia: A systematic review and meta-analysis. Front Immunol. 2023;14:1152457.
- [CrossRef] [PubMed] [Google Scholar]
- CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119:696-706.
- [CrossRef] [PubMed] [Google Scholar]
- CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995-1004.
- [CrossRef] [PubMed] [Google Scholar]
- CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy. 2017;9:1115-25.
- [CrossRef] [PubMed] [Google Scholar]
- A bump in the road: How the hostile AML microenvironment affects CAR T cell therapy. Front Oncol. 2020;10:262.
- [CrossRef] [PubMed] [Google Scholar]
- Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-Cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112-21.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161:389-401.
- [CrossRef] [PubMed] [Google Scholar]
- Acute myeloid leukemia - cancer stat facts. 2022. Available from: https://seer.cancer.gov/statfacts/html/amyl.html [Last accessed on 2024 Aug 12]
- [Google Scholar]
- First-inhuman CLL1-CD33 compound CAR (cCAR) T cell therapy in relapsed and refractory acute myeloid leukemia In: Proceedings of the 25th EHA annual congress. 2020. p. :12.
- [Google Scholar]
- Overcoming intrinsic resistance of cancer cells to CAR T-cell killing. Clin Cancer Res. 2021;27:6298-306.
- [CrossRef] [PubMed] [Google Scholar]
- CD38-directed CAR-T cell therapy: A novel immunotherapy strategy for relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2021;14:82.
- [CrossRef] [PubMed] [Google Scholar]
- NKG2D-based chimeric antigen receptor therapy induced remission in a relapsed/refractory acute myeloid leukemia patient. Haematologica. 2018;103:e424-6.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23:184-91.
- [CrossRef] [PubMed] [Google Scholar]
- First-in-man CD123-specific chimeric antigen receptor-modified T cells for the treatment of refractory acute myeloid leukemia. Blood. 2015;126:3778.
- [CrossRef] [Google Scholar]
- Bispecific and split CAR T cells targeting CD13 and TIM3 eradicate acute myeloid leukemia. Blood. 2020;135:713-23.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424-47.
- [CrossRef] [PubMed] [Google Scholar]
- Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705-16.
- [CrossRef] [PubMed] [Google Scholar]
- Current status and future clinical directions in the prevention and treatment of relapse following hematopoietic transplantation for acute myeloid and lymphoblastic leukemia. Bone Marrow Transplant. 2019;54:6-16.
- [CrossRef] [PubMed] [Google Scholar]
- A novel approach for relapsed/refractory FLT3mut+ acute myeloid leukaemia: Synergistic effect of the combination of bispecific FLT3scFv/NKG2D-CAR T cells and gilteritinib. Mol Cancer. 2022;21:66.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of trends and prognosis over time in patients with AML relapsing after allogeneic hematopoeitic cell transplant reveals improved survival for young patients in recent years. Clin Cancer Res. 2020;26:6475-82.
- [CrossRef] [PubMed] [Google Scholar]
- Comparable outcomes among unmanipulated haploidentical, matched unrelated, and matched sibling donors in BU-based myeloablative hematopoietic stem cell transplantation for intermediate and adverse risk acute myeloid leukemia in complete remission: A single-center study. Ann Hematol. 2021;100:1579-91.
- [CrossRef] [PubMed] [Google Scholar]
- Haploidentical age-adapted myeloablative transplant and regulatory and effector T cells for acute myeloid leukemia. Blood Adv. 2021;5:1199-208.
- [CrossRef] [PubMed] [Google Scholar]
- Azacitidine for pre-emptive treatment of measurable-residual disease in MDS/AML patients at high risk of hematological relapse: Results of the second cohort of the RELAZA2 trial. Blood. 2019;134:644.
- [CrossRef] [Google Scholar]
- Prediction of response and survival following treatment with azacitidine for relapse of acute myeloid leukemia and myelodysplastic syndromes after allogeneic hematopoietic stem cell transplantation. Cancers (Basel). 2020;12:2255.
- [CrossRef] [PubMed] [Google Scholar]
- Sequential administration of low dose 5-azacytidine (AZA) and donor lymphocyte infusion (DLI) for patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) in relapse after allogeneic stem cell transplantation (SCT): A prospective study from the Belgian Hematology Society (BHS) Bone Marrow Transplant. 2022;57:116-8.
- [CrossRef] [PubMed] [Google Scholar]







