Translate this page into:
Cold agglutinin syndrome secondary to Mycoplasma pneumoniae
*Corresponding author: Bilal Shoeb Kazi, Department of Clinical Hematology, Apollo Multi-Speciality Hospital, Kolkata, West Bengal, India. kazibilal22@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kazi BS, Maurya A, Chakrapani A, Rumani I. Cold agglutinin syndrome secondary to Mycoplasma pneumonia. J Hematol Allied Sci. 2024;4:46-7. doi: 10.25259/JHAS_42_2023
Abstract
A 47-year-old female presented with dyspnea, dry cough, and fatigue for ten days. On examination, she had severe pallor and bilateral crepts in the lungs. Complete blood count (CBC) showed a hemoglobin of 7.3 g/dL, reticulocyte count of 5.6%, and chest X-ray was normal. Her extended Direct Coombs test (DCT) showed C3d +3 and cold agglutinin titer of 1:256 at 4°C. A comprehensive respiratory panel showed positivity for Mycoplasma pneumonia. Thus, a diagnosis of cold agglutinin syndrome secondary to M. pneumonia was made, and she was started on injection doxycycline 100 mg IV twice a day, following which her symptoms and anemia improved without transfusion support.
Keywords
Cold agglutinin syndrome
Mycoplasma pneumoniae
Cold agglutinin
DCT
INTRODUCTION
Cold agglutinin syndrome (CAS) is a rare type of acquired auto-immune hemolytic anemia (AIHA). Similar to warm AIHA (wAIHA), CAS results from a pathogenic autoantibody that targets an antigen that is found on normal red blood cells (RBC). Unlike in wAIHA (where an IgG antibody causes hemolysis), the offending antibody in CAS is an IgM antibody.
CASE REPORT
A 47-year-old female presented to the emergency room of a tertiary health care hospital with complaints of shortness of breath, dry cough, fever, and fatigue for ten days. She had no history of similar complaints or any blood transfusion in the past. On further interrogation, she revealed that the previous hospital from where she was referred was unable to transfuse her packed red blood cells (RBCs) due to discrepancies in forward and reverse blood grouping. On examination, she had severe pallor, bilateral coarse crepts in the lungs, no icterus, organomegaly, and lymphadenopathy. CBC showed hemoglobin (Hb) of 7.3 gm/dL, TLC: 26 × 109/L, and platelets: 200 × 109/L. Her reticulocyte count was 5.6%, and her LDH was 397 IU/dL. Chest X-ray did not reveal any significant abnormality. Based on anemia and elevated reticulocyte count, a Direct Coombs test (DCT) was ordered. Her DCT was +3, and extended DCT [Figure 1] was only positive for C3d (+3). In view of isolated C3d positivity, a cold agglutinin test was ordered. Her cold agglutinin was positive with a titer of 1:256 at 4°C. Based on a positive cold agglutinin test and a history of dyspnea and dry cough, Mycoplasma pneumonia was suspected. A comprehensive respiratory panel from her throat and nasopharyngeal swab was sent, which was positive for M. pneumonia. Thus, a diagnosis of cold agglutinin syndrome (CAS) was made. She was started on injection doxycycline 100 mg IV twice a day. After two days of antibiotic, her cough and dyspnea showed improvement and her Hb improved to 9 g/dL without any transfusion support.
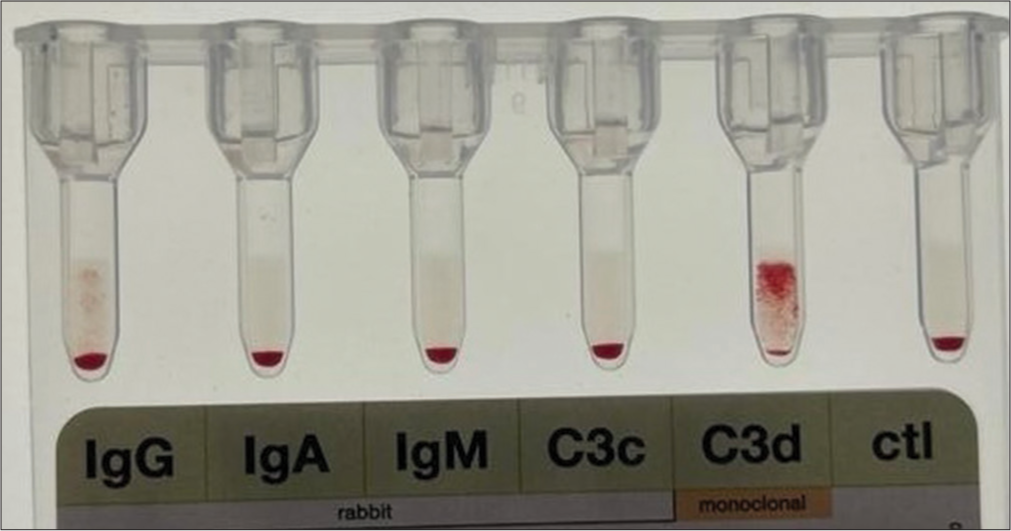
- Extended Direct Coombs Test (DCT) showing C3d positivity.
DISCUSSION
M. pneumoniae infection outside the respiratory tract can occur independently or in conjunction with respiratory tract disease. M. pneumoniae causes hemolysis (CAS) in 60% cases and is typically mild or subclinical.[1] During the course of infection, Mycoplasma induces a modification in the I antigen on the RBC membrane. This, in turn, leads to the formation of an immunoglobulin M autoantibody against this antigen and immune-mediated hemolysis.[2,3] Majority of patients have self-limited hemolysis, with transfusion or immunosuppressive treatment seldomly required. Seldomly, hemolysis can be severe and lethal, particularly in patients with underlying hematologic disorders such as sickle cell disease.[4]
CONCLUSION
Our case highlights the importance of having a low threshold for suspecting CAS secondary to M. pneumoniae in a patient presenting with dyspnea, dry cough, and anemia. Furthermore, CAS secondary to M. pneumoniae causes mild hemolysis and can be managed with antibiotics with seldom requirement of transfusion and immunosuppressants.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent is not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- How I treat autoimmune hemolytic anemia. Blood. 2017;129:2971-9.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26:107-15.
- [CrossRef] [PubMed] [Google Scholar]
- Severe hemolytic anemia and excessive leukocytosis masking mycoplasma pneumonia. Ann Hematol. 2001;80:180-2.
- [CrossRef] [PubMed] [Google Scholar]
- Anemic crisis due to Mycoplasma pneumoniae complication in sickle cell patients. Saudi Med J. 2009;30:157-8.
- [Google Scholar]






