Translate this page into:
Epstein-Barr virus positive mucocutaneous ulcer – A diagnostic challenge
*Corresponding author: Sohaila Fatima, Department of Pathology, King Khalid University, Abha, Saudi Arabia. sohailafatima@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Osman TA, Fatima S. Epstein-Barr virus-positive mucocutaneous ulcer – A diagnostic challenge. J Hematol Allied Sci. 2023;3:71-3. doi: 10.25259/JHAS_8_2023
Abstract
A significant portion of the global population carries the asymptomatic Epstein-Barr virus (EBV). Transfusions of blood and blood derivatives, organ and tissue transplantation, and oropharyngeal secretions are the main routes of transmission. The World Health Organization recently recognized the EBV mucocutaneous ulcer (EBVMU) as a provisional pathological entity in its classification of hematopoietic and lymphoid tissues. It affects patients who are elderly or immunosuppressed. Histologically, it may be difficult to distinguish from Hodgkin lymphoma (HL). Although skin or mucosa are uncommon sites of HL involvement, diagnosis at these sites should be made with extreme caution. The prognosis for EBVMU is good; cases regress on their own or after immunosuppressive therapy is reduced. We present a 67-year-old woman with a non-healing oral cavity ulcer which was diagnosed as EBV-positive mucocutaneous ulcer.
Keywords
Epstein-Barr virus-positive
Mucocutaneous ulcer disease
Lymphoproliferative disorder
INTRODUCTION
A considerable proportion of the world’s population is estimated to be an asymptomatic carrier of the common Epstein-Barr virus (EBV).[1] EBV has been related to infectious mononucleosis, lymphoma, post-transplant lymphoproliferative disease, and nasopharyngeal malignancy. EBV mucocutaneous ulcer (EBVMU) is a newly recognized provisional pathological entity within the category of mature B-cell neoplasms in the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid organs.[2] EBVMU affects patients who have human immunodeficiency virus infection or immunosuppression as a result of age, iatrogenic causes, or both. The infiltrate beneath the ulcer is a polymorphic mixture of cells with a few dispersed Reed-Sternberg (RS)-like cells, according to histology. Although skin or mucosa involvement in HL is uncommon, a diagnosis at these sites should be approached with extreme caution. EBVMU has a good prognosis, with the majority of cases regressing spontaneously or with a reduction in immunosuppressive treatment. A 67-year-old female presented with a non-healing ulcer at the left vestibule and alveolar mucosa for 6 months, which was diagnosed as an EBV-positive mucocutaneous ulcer. Brentuximab was started on her and she is responding to it.
CASE REPORT
A 67-year-old female presented with a non-healing oral ulcer for 6 months. On examination, an ulcer at the left vestibule and alveolar mucosa was noted. She had a history of dermatomyositis for 10 years and had been receiving Rituximab. Post-contrast computed tomography (CT) examination of the neck showed a left mandibular alveolar soft-tissue mass lesion associated with multiple mildly enlarged submental, left submandibular, left intraparotid, and bilateral upper deep cervical lymph nodes. Magnetic resonance imaging of the head and neck revealed a left extra osseous soft-tissue mass lesion extending along the left mandibular length with focal osseous erosion and extension to submental, left submandibular, and left sublingual spaces with cervical lymphadenopathy. Our patient had repeated biopsies (three) from the ulcer and it was initially diagnosed as a non-specific ulcer with no evidence of squamous cell carcinoma. The third biopsy of the ulcer showed surface ulceration with an underlying dense polymorphic cellular infiltrate composed of lymphocytes, histiocytes, and scattered large atypical cells with RS-like morphology [Figure 1]. A panel of immuno-stains was performed and the large atypical cells were positive for CD30, CD19, PAX5, MUM1, and EBV (latent membrane protein, LMP) and negative for cytomegalovirus, herpes simplex virus type 1, CD20, CD10, CD15, CD3, B-cell lymphoma 6 (BCL6), and CD68 [Figure 2]. The overall morphologic and immune-stain findings were consistent with EBV-positive mucocutaneous ulcer.

- (a-d) Section from oral mucosa showing surface ulceration with an underlying dense polymorphic cellular infiltrate composed of lymphocytes, histiocytes, and scattered large atypical cells with Reed-Sternberg-like morphology (Hematoxylin and Eosin: a and b ×10, c: ×20, d: ×40).
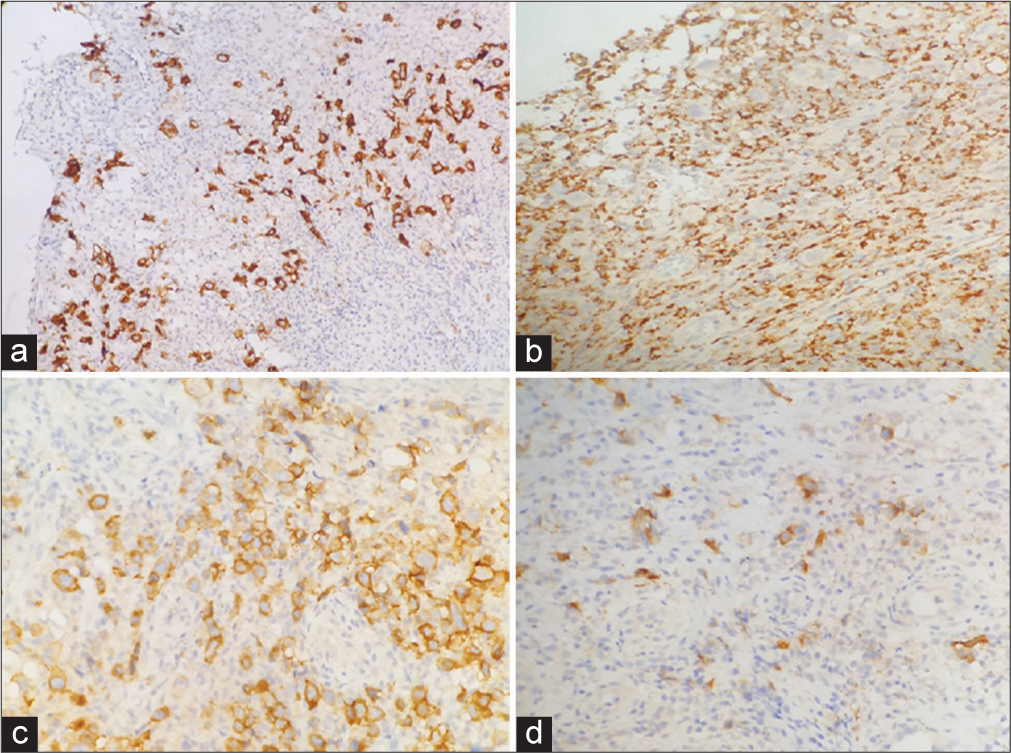
- (a) Immunohistochemical study. (a) Showing CD30 positivity in large atypical cells (CD30 ×10). (b) Showing CD68 negativity in large atypical cells (CD68 ×20). (c) Showing CD19 positivity in large atypical cells (CD19 ×20). (d) Showing Epstein-Barr virus (EBV)-latent membrane protein (LMP) positivity in large atypical cells (EBV-LMP ×20).
DISCUSSION
EBV belongs to the Herpesviridae family and human herpesvirus 4 species. The Centers for Disease Control and Prevention reports that 95% of adults aged 35–40 are EBV carriers. It is mostly transmitted by contact with virus-containing oropharyngeal secretions. Blood and blood derivative transfusions, as well as organ and tissue transplantation, are other modes of administration.[3] It selectively infects B-cells by attaching the viral envelope glycoprotein gp350/220 to B-cell CD21 (complement receptor type 2).[4] Although an initial lytic infection can occur, viral latency is more common, which promotes lymphocyte survival and multiplication. EBVMU is a newly identified B cell lymphoproliferative entity induced by latent EBV infection and marked by isolated oropharyngeal, gastrointestinal tract (GIT), and skin ulcerations.[5]
At present, EBVMU is classed as a low-grade or pseudo-malignant lesion.[6] It is listed as a tentative entity under hematological and lymphoid cancers in the 2017 WHO classification.[2] EBVMU was first described and thought to be a unique clinical entity in 2010. It is a rare lymphoproliferative illness characterized by oropharyngeal mucosal ulcers, cutaneous, and GIT ulcers with mixed hematolymphoid infiltration, and Hodgkin-like morphologic and immunohistochemical features.[7] EBVMUs are commonly identified in the oral cavity and pharynx (including the tonsils), presumably due to EBV release into saliva.[6] Although there is localized regional lymphadenopathy, there is no evidence of systemic lymphadenopathy, hepatosplenomegaly, or bone marrow involvement. Regional lymph node reactive hyperplasia is possible.[2] Iatrogenic immunosuppression (56%), advanced age-related immunosenescence (40%), and primary immunosuppression (4%) have all been associated with this disease.[5]
Histologically, the infiltrate that encloses the ulcer is made up of a polymorphic mixture of eosinophils, lymphocytes, histiocytes, plasma cells, and lymphocytes. A few atypical immunoblasts with morphology resembling Hodgkin’s or RS-like cells also exist in the infiltrate. In addition, noticed are surface ulceration and focal necrosis. The large B cells, typically express CD20 in a strong to weak and heterogeneous manner. These cells show positive OCT2 and PAX5 expression. They exhibit an activated-B-cell phenotype, are CD30-positive, CD10- and BCL6-negative, and IRF4/MUM1-positive. CD15 is exhibited in almost 50% of cases. One of the viral gene products essential for B-cell transformation is LMP1,[8] which is frequently positive in transformed cells, indicating the presence of EBV,[2] and is consistently positive in all tested samples. Diffuse large BCL NOS and polymorphic post-transplant lymphoproliferative disease are the closest differentials.
Each patient’s disease progresses differently, and some exhibit full clinical improvement when their immunosuppressive medications are decreased. Others, however, may have chronic, debilitating EBVMU that requires aggressive treatment with CD20- or CD30-directed antibody therapy, local radiation therapy, local surgical excision, systemic chemotherapy, or a combination of these therapies. In 93% of cases of the disease, local or systemic therapy results in an excellent and long-lasting response. (Follow-up reports on durability ranged from 3 to 111 months). The prognosis is largely favorable with EBVMU. Relapsing and remitting disease without progression has been mentioned in a small number of case studies.[9] There is no expert opinion and treatment decisions are not supported by evidence.[5] Relapsing and remitting disease without progression has been described in some case reports.[9] Rituximab, an anti-CD20 monoclonal antibody, was being administered to our patient at the time of the diagnosis of EBVMU, which indicates a failure of response to this treatment. It was regarded as a neoplastic lesion eroding bone based on radiological investigations. The monoclonal antibody brentuximab, which targets the CD30 antigen on the surface of cells, was started on her.
CONCLUSION
The EBVMU is a newly identified provisional pathological entity that is classified as a lymphoproliferative disorder. It is a challenging diagnosis that calls for a high level of clinical suspicion and confirmatory histopathology to rule out other close differentials. There is no specific therapy and no expert opinion. EBVMU has a good prognosis, cases regress on their own or when immunosuppressive therapy is reduced. Radiation, chemotherapy, or other localized treatments have reportedly been used to treat persistent cases.
Declaration of patient consent
Patient’s consent was not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- EBV-positive mucocutaneous ulcer In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539738 [Last accessed on 2022 Sep 26]
- [Google Scholar]
- EBV-positive mucocutaneous ulcer. WHO classification of tumors of hematopoietic and lymphoid tissues In: Swerdlow SH, Campo E, Harris NL, Elaine S, Jaffe ES, Pileri SA, eds. World Health Organization classification of tumors (4th ed). Lyon, France: IARC; 2017.
- [Google Scholar]
- Epstein-Barr virus: general factors, virus-related diseases and measurement of viral load after transplant. Rev Bras Hematol Hemoter. 2011;33:383-8.
- [CrossRef] [PubMed] [Google Scholar]
- Epstein-Barr virus receptor of human B lymphocytes in the C3d receptor CR2. Proc Natl Acad Sci USA. 1984;81:4510-4.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic and therapeutic challenges of EBV-positive mucocutaneous ulcer: A case report and systematic review of the literature. Exp Hematol Oncol. 2015;5:13.
- [CrossRef] [PubMed] [Google Scholar]
- Epstein-Barr virus-positive mucocutaneous ulcer: A unique and curious disease entity. Int J Mol Sci. 2021;22:1053.
- [CrossRef] [PubMed] [Google Scholar]
- EBV positive mucocutaneous ulcer-a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405-17.
- [CrossRef] [PubMed] [Google Scholar]
- Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A. 1993;90:9150-4.
- [CrossRef] [PubMed] [Google Scholar]
- EBV-positive B-cell proliferations of varied malignant potential: 2015 SH/EAHP workshop report-part 1. Am J Clin Pathol. 2017;147:129-52.
- [CrossRef] [PubMed] [Google Scholar]






