Translate this page into:
From gut to blood: How microbiome metabolites orchestrate platelet function
*Corresponding author: Rahul Garg, Department of Medicine, F H Medical College, Agra, Uttar Pradesh, India. gargrahul27@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Garg R. From gut to blood: How microbiome metabolites orchestrate platelet function. J Hematol Allied Sci. 2025;5:6-10. doi: 10.25259/JHAS_65_2024
Abstract
The gut microbiome’s role in human health has gained significant attention, particularly regarding its metabolites’ influence on platelet function and cardiovascular health. This comprehensive review examines the complex relationship between gut microbial metabolites and platelet activity, focusing on key compounds such as trimethylamine N-oxide, 2-methylbutyrylcarnitine, and phenylacetylglutamine. These metabolites affect platelet function through both direct mechanisms, involving specific receptor interactions and calcium signaling pathways, and indirect effects through systemic inflammation. Recent research has revealed that elevated levels of these metabolites correlate with increased cardiovascular risk and altered response to antiplatelet therapy. Understanding these interactions opens new avenues for therapeutic intervention, including targeted metabolic pathway inhibition and personalized medicine approaches. While significant progress has been made, continued research is needed to fully elucidate the complex network of microbiome-platelet interactions and develop effective therapeutic strategies.
Keywords
2-methylbutyrylcarnitine
Microbiome
Phenylacetylglutamine
Platelets
Trimethylamine N-oxide
INTRODUCTION
The human gut microbiome, comprising trillions of microorganisms, has been increasingly recognized as a vital contributor to human health and disease. These microorganisms participate in numerous metabolic processes, producing a diverse array of bioactive compounds that can enter the circulation and affect multiple organ systems.[1] In recent years, substantial evidence has accumulated demonstrating the profound influence of gut microbial metabolites on cardiovascular health and, specifically, platelet function.
Platelets, while primarily known for their role in hemostasis, are increasingly understood to be complex cellular mediators involved in inflammation, immunity, and cardiovascular disease progression. The interaction between gut microbial metabolites and platelet function represents a fascinating intersection of microbial ecology and cardiovascular physiology.[2] This review comprehensively examines the current understanding of how gut microbial metabolites influence platelet function and their implications for cardiovascular health and disease.
KEY GUT MICROBIAL METABOLITES AFFECTING PLATELET FUNCTION
Trimethylamine N-oxide (TMAO)
TMAO has emerged as one of the most well-studied gut microbial metabolites affecting platelet function. Derived from dietary precursors such as choline, phosphatidylcholine, and L-carnitine, TMAO is produced through a two-step process involving initial bacterial metabolism followed by host liver oxidation.[1,3] Research by Zhu et al.[3] demonstrated that TMAO directly enhances platelet hyperreactivity and thrombosis potential through multiple mechanisms.
In their groundbreaking study, exposure to TMAO led to increased platelet responsiveness to multiple agonists through augmented calcium release from intracellular stores. This heightened platelet reactivity was observed both in vitro and in vivo, with dietary TMAO supplementation leading to shorter occlusion times in arterial thrombosis models.[3] Furthermore, clinical studies have shown strong correlations between elevated plasma TMAO levels and increased risk of thrombotic events.[1]
2-Methylbutyrylcarnitine (2MBC)
Recent research has identified 2MBC as a novel gut microbial co-metabolite with significant implications for thrombosis. Huang et al.[4] demonstrated that 2MBC exerts its prothrombotic effects through direct binding to and activation of platelet integrin α2β1. This discovery represents a previously unknown mechanism through which gut microbiota influence thrombotic risk and suggests new therapeutic targets for managing thrombotic disorders.
Phenylacetylglutamine (PAG)
PAG has emerged as another significant gut microbial metabolite with important cardiovascular effects. Krishnamoorthy et al.[5] have shown that PAG influences various aspects of cardiovascular function, including potential effects on platelet activity through both direct and indirect mechanisms. The role of PAG in cardiovascular health highlights the diverse ways in which gut microbial metabolites can influence platelet function and thrombotic risk.
MECHANISMS OF ACTION
Direct metabolite-platelet interactions
The mechanisms through which gut microbial metabolites influence platelet function are diverse and complex [Figure 1], involving multiple pathways and signaling cascades.[2,6] TMAO, one of the most well-studied metabolites, enhances platelet responsiveness through several distinct pathways that work in concert to promote platelet activation and aggregation. Research has demonstrated that TMAO increases calcium mobilization from intracellular stores, which serves as a crucial initial step in platelet activation. This calcium mobilization is accompanied by enhanced stimulus-dependent release of platelet granules, leading to amplification of the platelet response. In addition, TMAO potentiates platelet adhesion and aggregation responses, making platelets more responsive to various physiological agonists. Studies have also revealed that TMAO influences platelet function through alterations in mitochondrial function and energy metabolism, suggesting a complex interplay between cellular energetics and platelet reactivity.[3]
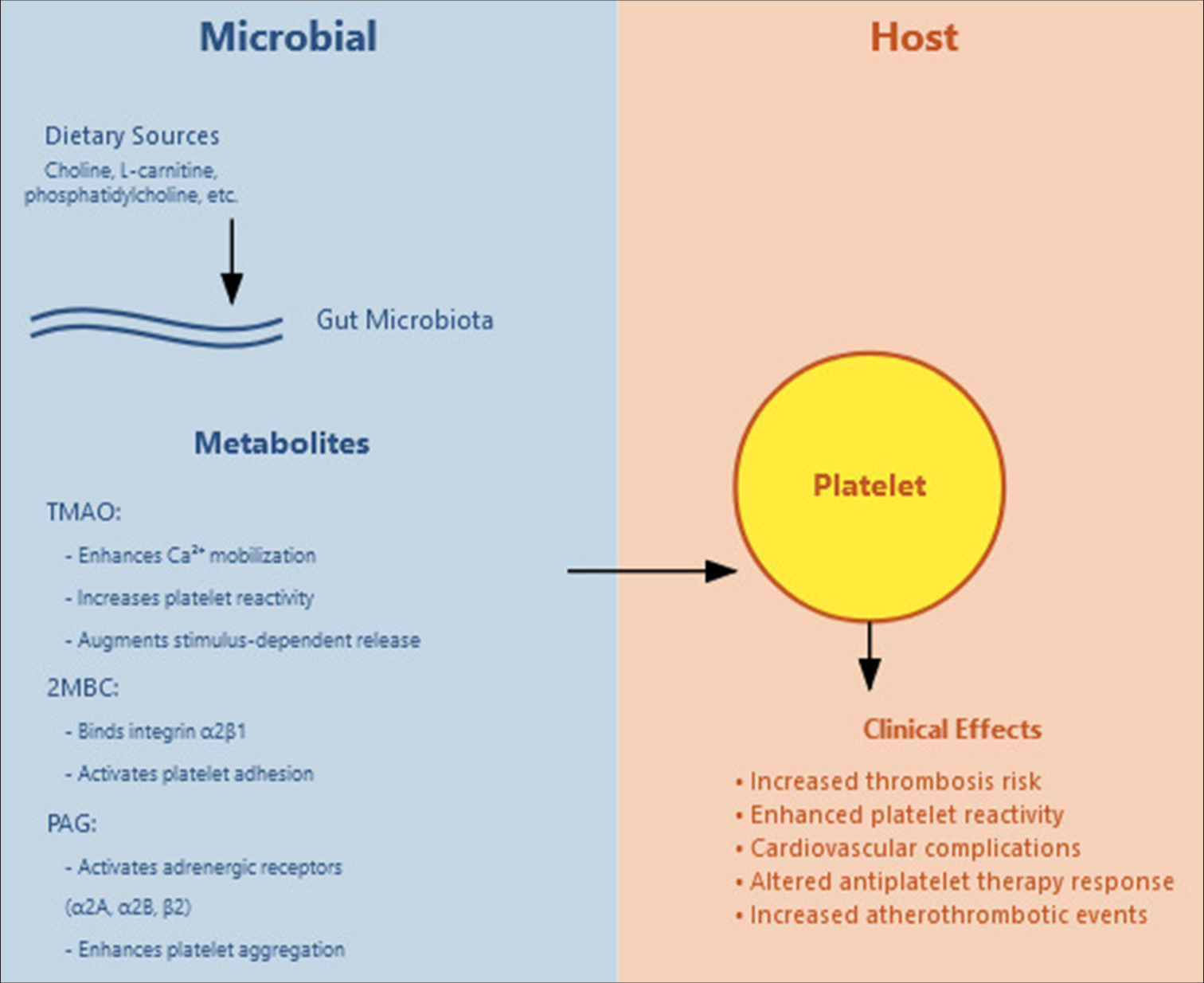
- Gut microbial metabolites and their effect on platelets. TMAO: Trimethylamine N-oxide, 2MBC: 2-methylbutyrylcarnitine, PAG: Phenylacetylglutamine.
2MBC, another significant metabolite, operates through a distinct mechanism that primarily involves direct binding to and activation of integrin α2β1 on platelets.[4] This interaction triggers a cascade of intracellular events that result in enhanced platelet activation and increased adhesion to collagen. The specificity of this interaction with integrin α2β1 represents a novel pathway through which gut microbiota can influence thrombotic potential. The binding of 2MBC to this integrin leads to elevated thrombotic potential through altered platelet signaling pathways, demonstrating how specific metabolite-receptor interactions can have profound effects on platelet function.
PAG exhibits its effects through interaction with multiple G-protein coupled receptors, specifically the α2A, α2B, and β2-adrenergic receptors.[5] This metabolite’s influence on platelet function is characterized by enhanced platelet adhesion to collagen and increased stimulus-dependent platelet aggregation in response to various agonists. Research has shown that PAG dose-dependently enhances sub-maximal adenosine diphosphate (ADP)-stimulated P-selectin surface expression and glycoprotein IIb/IIIa activation.[7] These changes in platelet surface protein expression and activation state contribute to an overall enhancement of platelet responsiveness, potentially increasing thrombotic risk in individuals with elevated PAG levels.
Indirect effects through systemic inflammation
Beyond their direct interactions with platelets, gut microbial metabolites exert significant influence on platelet function through broader effects on systemic inflammation and vascular health.[8] These metabolites participate in complex regulatory networks that modulate the production of inflammatory mediators throughout the body. The resulting changes in the inflammatory environment can significantly impact platelet function and reactivity. In addition, these metabolites induce alterations in endothelial function, which can create conditions that promote platelet activation and adhesion.
The systemic effects of gut microbial metabolites extend to broader changes in the overall inflammatory state of the vasculature. These changes can create a pro-thrombotic environment that enhances platelet reactivity and increases the risk of thrombotic events. Furthermore, these metabolites can modify platelet-leukocyte interactions, leading to the formation of platelet-leukocyte aggregates that play important roles in both thrombosis and inflammation. The complex interplay between these direct and indirect mechanisms creates a sophisticated network of regulatory pathways through which gut microbial metabolites can influence platelet function and thrombotic risk.[2,6]
CLINICAL IMPLICATIONS
Cardiovascular disease risk
The relationship between gut microbial metabolites and platelet function has significant implications for cardiovascular disease risk assessment and management. Studies have consistently shown that elevated levels of certain metabolites, particularly TMAO, correlate with increased cardiovascular event risk.[1,6] This association appears to be independent of traditional cardiovascular risk factors, suggesting that gut microbial metabolites represent a novel and potentially modifiable risk factor for cardiovascular disease.
Thrombotic disorders
Recent research has revealed important connections between gut microbiota composition, metabolite production, and thrombotic disorders. Zhang et al.[9] demonstrated significant alterations in both gut microbiome composition and metabolomic profiles in patients with primary immune thrombocytopenia. These findings suggest that disruptions in the gut microbiome-platelet axis may contribute to the pathogenesis of various thrombotic disorders.
Antiplatelet therapy response
Perhaps one of the most clinically relevant discoveries has been the influence of gut microbiota on antiplatelet therapy efficacy. Zhang et al.[10] showed that gut microbiota can significantly affect the response to ticagrelor treatment in patients with ST-segment elevation myocardial infarction. This finding has important implications for personalized antiplatelet therapy and suggests that individual variations in gut microbiota composition might contribute to differences in treatment effectiveness.
THERAPEUTIC IMPLICATIONS
Targeting metabolic pathways
Understanding the specific pathways through which gut microbial metabolites influence platelet function opens new therapeutic possibilities. Current research suggests several promising approaches:[2,6]
Development of specific inhibitors targeting metabolite-platelet interactions
Dietary interventions to modify metabolite production
Microbiome-based therapeutic approaches
Novel drug delivery systems targeting the gut-platelet axis.
These approaches could potentially offer more targeted and effective treatments for thrombotic disorders while minimizing systemic side effects.
Personalized medicine approaches
The recognition of gut microbiota’s role in platelet function suggests the potential for personalized approaches to cardiovascular disease prevention and treatment.[1,11] This might include:
Microbiome profiling to assess cardiovascular risk
Tailored dietary interventions based on individual metabolite profiles
Personalized antiplatelet therapy selection
Targeted probiotic interventions.
Preventive strategies
The modifiable nature of the gut microbiome through diet and lifestyle interventions suggests several potential preventive strategies:[12]
Dietary modifications to reduce harmful metabolite production
Probiotic interventions to promote beneficial bacterial populations
Targeted approaches to modify specific metabolic pathways
Lifestyle interventions to maintain healthy gut microbiota.
CHALLENGES AND FUTURE RESEARCH DIRECTIONS
Methodological challenges
Several significant methodological challenges must be addressed to advance our understanding of gut microbiome-platelet interactions:
Standardization of metabolite measurement techniques
Development of more sophisticated models for studying microbiome-platelet interactions
Establishment of standardized protocols for microbiome analysis.
Knowledge gaps
Current research has identified several important knowledge gaps that require attention:
Understanding individual variation in metabolite production and response
Elucidating the complete network of metabolite-platelet interactions
Determining the relative importance of different metabolic pathways
Understanding the temporal dynamics of metabolite-platelet interactions
Identifying additional novel metabolites affecting platelet function.
Future research priorities
Future research efforts should focus on:
Large-scale longitudinal studies to establish causal relationships
Development of targeted therapeutic approaches
Investigation of potential synergistic effects between different metabolites
Understanding the impact of diet and lifestyle factors
Development of novel therapeutic agents targeting specific metabolic pathways.
CONCLUSION
The study of gut microbial metabolites’ impact on platelet function represents a rapidly evolving field with significant clinical implications. The identification of specific metabolites, such as TMAO, 2MBC, and PAG has enhanced our understanding of the gut microbiome-platelet axis in cardiovascular health. This knowledge presents new opportunities for therapeutic intervention and personalized medicine approaches. As research continues, we expect to uncover additional layers of complexity in these interactions, potentially leading to innovative treatments for cardiovascular disease and thrombotic disorders. This growing understanding may revolutionize our approach to managing cardiovascular health through microbiome-based interventions.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Gut microbiota and their metabolites in cardiovascular disease. Best Pract Res Clin Endocrinol Metab. 2021;35:101492.
- [CrossRef] [PubMed] [Google Scholar]
- Role of gut microbiota and their metabolites on atherosclerosis, hypertension and human blood platelet function: A review. Nutrients. 2021;13:144.
- [CrossRef] [PubMed] [Google Scholar]
- Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111-24.
- [CrossRef] [PubMed] [Google Scholar]
- Gut microbial co-metabolite 2-methylbutyrylcarnitine exacerbates thrombosis via binding to and activating integrin α2β1. Cell Metab. 2023;36:598-616.
- [CrossRef] [PubMed] [Google Scholar]
- Role of the gut bacteria-derived metabolite phenylacetylglutamine in health and diseases. ACS Omega. 2024;9:3164-72.
- [CrossRef] [PubMed] [Google Scholar]
- Role of gut microbial metabolites in cardiovascular diseases-current insights and the road ahead. Int J Mol Sci. 2024;25:10208.
- [CrossRef] [PubMed] [Google Scholar]
- A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180:862-77.
- [CrossRef] [PubMed] [Google Scholar]
- Bridging the gap between gut microbial dysbiosis and cardiovascular diseases. Nutrients. 2017;9:859.
- [CrossRef] [PubMed] [Google Scholar]
- Gut microbiome and metabolome were altered and strongly associated with platelet count in adult patients with primary immune thrombocytopenia. Front Microbiol. 2020;11:1550.
- [CrossRef] [PubMed] [Google Scholar]
- Gut microbiota induces high platelet response in patients with ST segment elevation myocardial infarction after ticagrelor treatment. eLife. 2022;11:e70240.
- [CrossRef] [PubMed] [Google Scholar]
- Gut microbes modulate platelet function and thrombosis risk. Nat Rev Cardiol. 2016;13:247.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary components and human platelet activity. Platelets. 2002;13:67-75.
- [CrossRef] [PubMed] [Google Scholar]






