Translate this page into:
Isolated fungal liver abscess in pediatric acute lymphoblastic leukemia – A case report
*Corresponding author: Reshma Benson, Department of Medical Oncology Haematology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India. drreshmabenson@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Benson R, Kumar K, Prasad A, Nath UK. Isolated fungal liver abscess in pediatric acute lymphoblastic leukemia – A case report. J Hematol Allied Sci. 2025;5:84-7. doi: 10.25259/JHAS_47_2024
Abstract
Diagnosis and management of invasive fungal infections pose a challenge to the treating physician in patients with hematological malignancies. The incidence is lesser in acute lymphoblastic leukemia (ALL), compared to the other hematological malignancies. The diagnosis is often delayed due to the non-specific clinical findings and negative cultures. Here, we report a case of B-cell ALL, on chemotherapy, diagnosed to have hepatic fungal abscess caused by Aspergillus fumigatus species. Interestingly, the patient did not have neither neutropenia nor any other systemic involvement of fungal infection at presentation. The case was successfully managed with percutaneous drainage and systemic antifungal therapy using voriconazole. Timely intervention helped in the diagnosis and proper management of the case, thus preventing a disseminated invasive fungal infection.
Keywords
Acute lymphoblastic leukemia
Antifungal
Aspergillus
Liver abscess
INTRODUCTION
Fungal infections are common cause of morbidity in immunocompromised patients, especially in hematological malignancies such as acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and post-hematopoietic stem cell transplant (HSCT) recipients. Fungal infections often pose a diagnostic and therapeutic challenge to physicians, due to the oftennonspecific symptoms and signs, difficulty in obtaining appropriate samples for microbiological diagnosis, and choosing effective antifungal agent, avoiding drug interactions and managing cytopenias. We present a rare case of B-cell ALL who initially presented with symptoms of cold agglutinin disease was diagnosed to have hepatic fungal abscess. The patient responded well to oral voriconazole therapy and is still under follow-up.
CASE REPORT
A 4-year-old boy, who is a follow-up case of high-risk precursor B-cell ALL in remission, on late reinduction phase of chemotherapy (Berlin Frankfurt Munster 2009 protocol), presented with history of high-grade fever, coryza, and cough followed by bluish discoloration of extremities for 3 days. On examination, he was afebrile, had tachycardia, pallor, and acrocyanosis. He had a tender hepatomegaly of 16 cm in size, with prominent right lobe involvement. The rest of the systemic examination was normal. On investigations, the blood sampling showed auto agglutination in room temperature. On analyzing, his samples warmed to 37°C, his hemoglobin was 5.1 g/dL, hematocrit of 14.3%, with a mean corpuscular volume of 118, mean corpuscular hemoglobin 42.1 pg, mean corpuscular hemoglobin concentration of 35.7 g/dL, and reticulocyte count was 19.59%. He had a total count of 42410 cells/mm3 (neutrophils 88%, lymphocytes 10%, and monocytes 2%) and a platelet count of 2.82 lakhs/mm3. The peripheral smear examination showed normocytic normochromic red blood cells (RBC) with agglutination and 30 nucleated RBCs/white blood cells. His direct antiglobulin test and auto control were positive (4+). The monospecific antibody testing showed the presence of C3d/C3b. The cold antibody titer (Anti I) was performed and was found to be 256 with thermal amplitude of 4–37°C. He also had elevated alkaline phosphatase (ALP) of 736 U/L and gamma-glutamyl transferase (GGT) of 800 U/L. The rest of the liver function (total bilirubin – 0.52 mg/dL, direct bilirubin – 0.19 mg/dL, serum glutamic oxaloacetic transaminase (SGOT) – 57 U/L, serum glutamic pyruvic transaminase (SGPT) – 16 U/L, total protein – 7.5 g/L, and albumin – 4.1 g/L) and renal function tests were within normal limits. Based on the diagnosis of cold autoimmune hemolytic anemia, urgent treatment was initiated with injection rituximab given at 375 mg/m2 over 2 days, prewarmed blood transfusion, injection methylprednisolone 1 mg/kg/day for 5 days, and broad-spectrum antibiotics. His hemoglobin improved to 11.3 g/dL, hematocrit was 32%, total count was 6240 cells/mm3 (neutrophil 63%, lymphocytes 34%, and monocytes 3%), and all RBC indices were normalized by day 5 of treatment. Before reinitiating chemotherapy, we further evaluated the patient for tender hepatomegaly. A computed tomography imaging of the abdomen revealed multiple peripherally enhancing hypodense lesions with hyperdense debris within, in the segments IVa, IVb, V, and III of the liver, suggestive of abscess [Figure 1]. The largest lesion was in segment IV b, measuring 4*2.1*1.8 cm, which had an exophytic component lesion of size 7*8.7*8 cm. The lesion on segment IVa was seen causing mass effect on the adjacent biliary tree with mild peripheral intrahepatic biliary radicle dilatation. There were no lesions in the lungs or any other abdominal organs. The patient was continued on antibiotics and started on injection metronidazole. The patient underwent percutaneous drainage of the largest lesion, which yielded around 30 mL of purulent material and the samples were sent for analysis. The analysis of the aspirate sample was sent for Cartridge Based Nucleic Acid Amplification Test for Mycobacterium tuberculosis, which was negative. The Echinococcus granulosus and Entamoeba histiolytica serology were negative. Gram stain and aerobic and anaerobic cultures did not reveal any pathogenic organisms. However, fungal stain revealed thin septate hyaline fungi, following which he was started on oral voriconazole 100 mg twice daily. Fungal culture reports followed later, which isolated Aspergillus fumigatus [Figure 2]. The patient was continued on oral voriconazole, which the patient tolerated well. His percutaneous drain was removed 14 days later, after a repeat imaging which showed reduction in size of all the lesions. During the time of discharge, his hepatomegaly had completely resolved, and his liver function test had begun to normalize (total bilirubin – 0.79 mg/dL, direct bilirubin – 0.13 mg/dL, SGOT – 162 U/L, SGPT – 78 U/L, ALP – 340 U/L, GGT – 556 U/L, total protein – 6.4 g/dL, and albumin – 3.5 g/dL). The patient was reinstated on chemotherapy. A repeat imaging showed complete resolution of all the liver lesions, oral voriconazole therapy was continued till 6 months and the patient is still under follow-up.
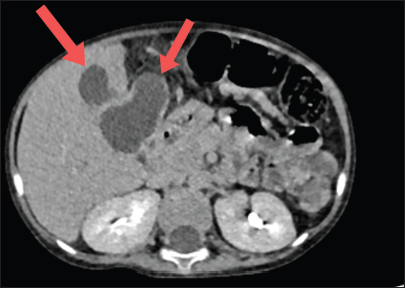
- Contrast enhanced computed tomography image of abdomen showing multiple hypodense lesions in the liver (red arrows).
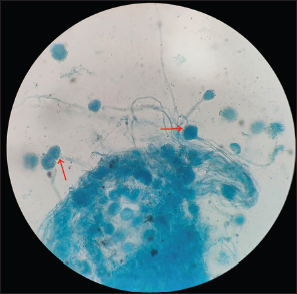
- Lactophenol cotton blue mount from the culture at 40× magnification showing growth of Aspergillus fumigatus species (red arrows).
DISCUSSION
Invasive fungal infections are increasing in prevalence in acute leukemia patients. In the past, before the introduction of fluconazole as prophylaxis for fungal infection, Candida species were the most common, but in the recent times, Aspergillus species have been more prevalent.[1] The diagnosis is often delayed due to the non-specific clinical findings and negative cultures. The risk of fungal infection is much higher in patients of AML, ALL with intensified treatment regimens, and HSCT patients. Guidelines suggest anti-mold prophylaxis in AML patients. Childhood ALL is considered to be at low risk for invasive fungal infections; however, prolonged steroid therapy is considered to be a risk factor.[2] In ALL, antifungal prophylaxis is only recommended only if neutropenia is expected to persist more than 10 days, due to lower incidence of fungal infections and the interaction between triazoles and vinca alkaloids used in ALL therapy.[3,4]
According to a large multicenter study, Aspergillus flavus is the most common cause of invasive aspergillosis in children.[5] Here, we reported a patient who was in reinduction phase of chemotherapy, not in neutropenia, diagnosed to have culture positive A. fumigatus hepatic abscess. He was successfully managed with percutaneous drain and systemic antifungal therapy with voriconazole.
In a retrospective clinical study of 58 patients of ALL from Germany by Gründahl et al., the rate of proven, probable, and possible fungal infections demonstrated was 3.4%, 8.6%, and 17.2% likelihood, respectively. They also report that the diagnosis of fungal infection had an impact on the total duration of hospitalization, which was 13 days more than patients with no fungal infection. In this study, the use of antifungal prophylaxis did not significantly affect the risk of fungal infection.[6]
Zwitserloot et al. have reported a similar case of disseminated aspergillosis caused by A. flavus, involving the lungs, thyroid gland causing abscess, and possible central nervous system (CNS) infection. The patient was managed with iv Caspofungin therapy.[7] Gottfredsson and Steingrímsdóttir has reported a case of adult ALL with pulmonary aspergillosis, which further disseminated to involve the CNS, kidneys, and liver. This patient was managed with IV liposomal amphotericin B.[8] Eroglu et al. have reported a case of a 3.5-year-old patient of relapsed T-cell ALL with aspergillosis of small bowel, proven by biopsy and managed with dual antifungal therapy and surgical resection.[9]
The various case reports of disseminated aspergillosis in ALL which were reviewed showed requirement of use of dual antifungals and involvement of other organ systems. The isolated involvement of the liver, without lung or other organ involvement, which was managed with single antifungal therapy has not yet been reported. This patient who was initially admitted for evaluation of cold agglutinin disease was incidentally detected to have tender hepatomegaly and evaluated for the same. There were no clinical symptoms such as fever, jaundice, or abdominal pain. Moreover, this patient was not neutropenic at the time of presentation, with no past history of fungal pneumonia. The duration of the hepatic abscess cannot be determined in this case. The early diagnosis and intervention have helped in managing the infection and continuing chemotherapy later. If undiagnosed, it would have probably lead to a disseminated Aspergillus infection later in the course of chemotherapy during neutropenia.
CONCLUSION
The timely diagnosis of fungal infection is very crucial in patients with ALL. In localized disease, the use of potent systemic antifungal therapy can cure the infection and prevent disseminated disease, which can otherwise be detrimental for patients with acute leukemia.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- How we treat invasive fungal diseases in patients with acute leukemia: The importance of an individualized approach. Blood. 2014;124:3858-69.
- [CrossRef] [PubMed] [Google Scholar]
- Towards a targeted, risk-based, antifungal strategy in neutropenic patients. Br J Haematol. 2000;110:273-84.
- [CrossRef] [PubMed] [Google Scholar]
- Practice guidelines for the diagnosis and management of aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1-60.
- [CrossRef] [PubMed] [Google Scholar]
- Primary prophylaxis of invasive fungal infections in patients with haematologic malignancies. 2014 update of the recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Ann Hematol. 2014;93:1449-56.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric invasive aspergillosis: A multicenter retrospective analysis of 139 contemporary cases. Pediatrics. 2008;121:e1286-94.
- [CrossRef] [PubMed] [Google Scholar]
- Invasive fungal diseases in patients with new diagnosed acute lymphoblastic leukaemia. Mycoses. 2020;63:1101-6.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated aspergillosis in an adolescent with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51:423-6.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated invasive aspergillosis in a patient with acute leukemia. Acta Biomed. 2006;77(Suppl 2):10-3.
- [Google Scholar]
- Invasive aspergillosis of the small bowel in a patient with acute lymphoblastic leukemia: Case report and a systematic review of the literature. J Tepecik Educ Res Hosp. 2022;32:481-7.
- [CrossRef] [Google Scholar]






