Translate this page into:
Levels of agreement between various serological and molecular tests for human immunodeficiency virus screening in blood donors
*Corresponding author: Dibyajyoti Sahoo, Department of Transfusion Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research, Puduchery, India. dib.jit@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Paudel P, Sahoo D, Singh R, Basavarajegowda A. Levels of agreement between various serological and molecular tests for human immunodeficiency virus screening in blood donors. J Hematol Allied Sci. doi: 10.25259/JHAS_8_2025
Abstract
Objectives
Many serological and molecular tests are available to reduce the spread of human immunodeficiency virus (HIV) transmission through blood transfusions. These include enzyme-linked immunosorbent assay (ELISA), rapid card tests, chemiluminescence, and nucleic acid testing. The level of agreement among all these test results has hardly been accessed. The aim of the present study was to evaluate the level of agreement among these commonly used tests.
Material and Methods
This is a cross-sectional study done from November 2021 to May 2023. Out of 5428 samples tested during the study period, 59 were reactive by fourth-generation ELISA. All these reactive samples were tested by another platform, that is, third-generation ELISA, enzyme-linked fluorescence assay (ELFA), polymerase chain reaction (PCR), and rapid card tests. An equal number of negative samples (i.e., 59) were also tested by all platforms.
Results
Out of all 59 samples that were reactive in 4th generation ELISA, only 17 came in 3rd generation ELISA. Of these, 13 only came to be positive in PCR. Similarly, 14 samples came to be positive in the ELFA test and rapid card test. Our study found the agreement to be 0.18, a fair agreement between third- and fourth-generation ELISA.
Conclusion
The present study has shown that although fourth-generation HIV ELISA has more sensitivity, false positives are present compared to third-generation ELISA. This study reflects the burden of HIV in the local population, and this result can be considered a preliminary step in quantifying the risk of Transfusion-Transmitted HIV and implementing different tests in blood donors.
Keywords
Blood donor
Enzyme-linked fluorescence assay
Enzyme-linked immunosorbent assay
Human immunodeficiency virus
Nucleic acid testing
Transfusion transmittable infection screening
INTRODUCTION
Blood donation is a voluntary procedure that can help to save many lives. The National Blood Policy was adopted by the Indian government in 2002. The provision of safe blood is an integral part of any blood transfusion services. With each donation, there is a chance of transmission of infections despite the stringent screening policy to exclude high-risk donors. Only five infections are screened in blood products because all infections cannot be screened practically. Some tests for other transfusion transmittable infections (TTIs) we do not do so as a policy since the prevalence is very low in our country.
All blood donations in India are required to undergo screenings for human immunodeficiency virus (HIV), hepatitis B virus, hepatitis C virus, syphilis, and malaria in accordance with the Drugs and Cosmetics Act. HIV is the most hazardous TTI among transfusion-transmitted diseases, primarily due to the high viral concentration in a unit of donor blood, the high rate of disease transmission following transfusion of an HIV-positive unit, the associated social stigma, and the lack of curative treatment.
Transmission of HIV infection is mainly caused by exposure to certain body fluids, for example, blood and blood products, genital secretion, and trans-placental route. HIV infection brought on by blood transfusion has been extensively documented.[1] According to a systemic review and meta-analysis, the overall pooled HIV seroprevalence rate among Indian blood donors was found to be 0.32 %.[2] With the frequent improvement in screening procedures and detection techniques, HIV infection rates through direct blood transfusion have been significantly reduced from 8% in the mid-1990s to 1% in 2009.[3]
Many platforms have evolved over time for the detection of HIV in blood donors. The spectrum includes rapid card tests, enzyme-linked immunosorbent assay (ELISA), chemiluminescence, and nucleic acid testing (NAT). Our study has been undertaken to analyze the positive test by 4th generation ELISA with third-generation ELISA, enzyme-linked fluorescence assay (ELFA), and polymerase chain reaction (PCR) test. This is the first of its kind in South India. The aim of the study was to compare levels of agreement between all these tests.
MATERIAL AND METHODS
Study design
This is a cross-sectional study on blood donors visiting the blood center at the Tertiary Care Center in South India. The study period was from November 2021 to May 2023. During routine screening, donors who came to donate blood from November 2021 to May 2023 were included in the study. HIV-reactive samples were detected using a fourth-generation ELISA. For comparison, an equal number of HIV-negative samples were also collected for tests in additional platforms. The inclusion criteria were all the blood samples collected after blood donation, while the exclusion criteria were insufficient samples and lysed samples due to storage.
Ethics statement
Ethical clearance was obtained from the Institutional Ethics Committee, on June 12, 2021, and the protocol number is JIP/IEC/2021/0117. A waiver of consent was granted by the Institutional Ethics Committee.
Sample size calculation
Sample size is estimated with an expected agreement between third-generation and fourth-generation ELISA which is detecting HIV as 0.95 with a population agreement of 0.40 at a 5% level of significance and 80% power. The estimated sample size was 53. An equal number of negatives for HIV was included in the study on the sample. To obtain 53 positive cases with an expected positivity rate of 0.36, 14,722 donors attending the blood donation were screened. The method used for calculation was the sample size for agreement on categorical variables.
The initial estimate was 53 each in positive and negative on the sample. Considering 10% samples for wastage and standardization of kits, the total sample size was 59 in each group.
Study Procedure
The steps of the procedure has been explained in Chart 1, below.
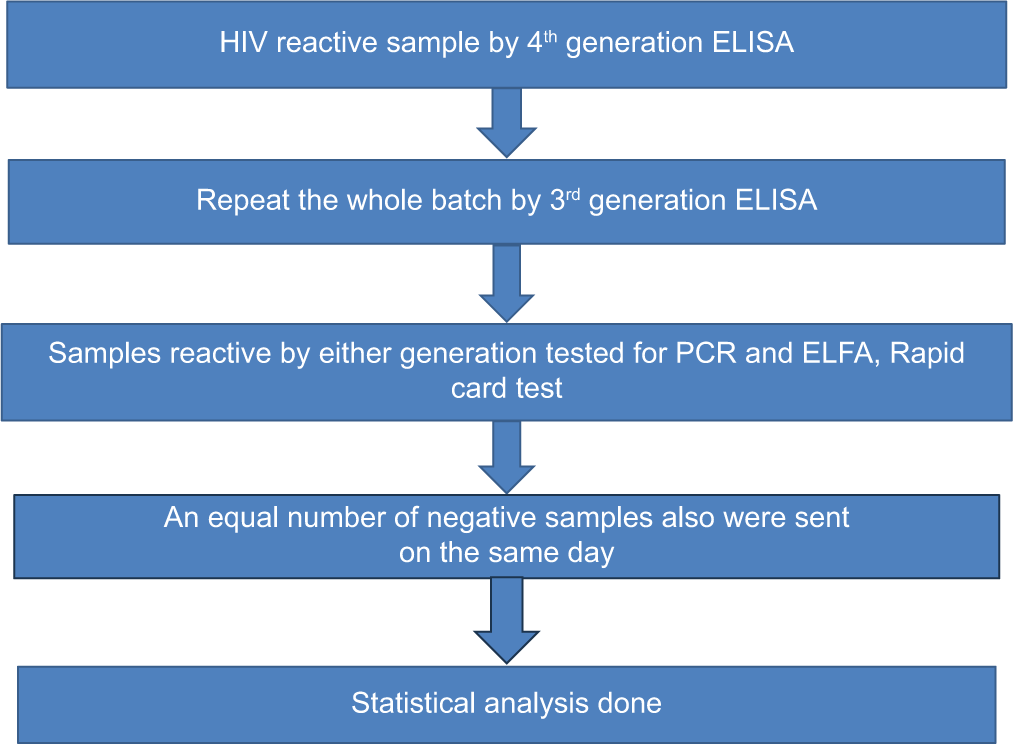
- Study procedure. ELISA: Enzyme-linked immunosorbent assay, ELFA: Enzyme-linked fluorescent assay, PCR: Polymerase chain reaction.
Sampling
All the blood donors during the study period were screened for HIV. The sampling method was convenient sampling. After donation, 5 mL of whole blood was collected in a clot activator tube for routine TTI testing. After TTI testing, all those samples that were reactive by fourth-generation HIV ELISA were included in the study. Third-generation ELISA, ELFA, PCR, and rapid tests were run on the same/next day of the reactive sample obtained by fourth-generation ELISA. All lysed samples due to storage were excluded from the study. Donor data such as blood group, occupation, education, and address were taken from the donor register.
For fourth- and third-generation ELISA testing, Qualisa HIV (Tulip, goa) was used. For ELFA testing,[4] we used Mini-Vidas instruments (Biomerieux, USA). The ELFA test simultaneously detects HIV-1 p24 antigen, anti-HIV-1 total immunoglobulins (Groups M and O), and anti-HIV-2 total immunoglobulins in human serum or plasma (lithium heparin or ethylene diamine tetra acetic acid [EDTA]). The kit used for PCR was Qiagen (Hilden, Germany). Real-time 2-step PCR was performed with a standardized method. For rapid testing, we used rapid HIV 1 and 2 card (Biolab Diagnostic Pvt Ltd, Mumbai, India) based on immunochromatography principles. Both PCR and ELFA were done in the Microbiology department, while ELISA and rapid tests were performed in the blood center.
Statistical analysis
All data was entered in Microsoft Excel and analyzed using the Statistical Package for the Social Sciences version 22. The data on categorical variables such as gender, blood group, occupation, and HIV status using third- and fourth-generation ELISA and PCR were expressed as frequency and percentages. Quantitative variables like age were expressed as mean with standard deviation or median with interquartile range. All statistical analysis was carried out at a 5% level of significance, and p-value < 0.05 was considered as significant.
κ – Cohen’s Kappa coefficient
We used Cohen’s Kappa coefficient[5] to see the level of agreement between various tests. The value of kappa may be interpreted as follows.
0: No agreement
0.10–0.20: Slight agreement
0.21–0.40: Fair agreement
0.41–0.60: Moderate agreement
0.61–0.80: Substantial agreement
0.81–0.99: Near perfect agreement
1: Perfect agreement.
RESULTS
A total of 118 ELISA reactive and negative group were analyzed in the study [Table 1]. Out of 59 reactive samples that were initially reactive with 4th-generation ELISA, only 17 came reactive in 3rd-generation ELISA [Table 2]. The prevalence of HIV by the fourth-generation was found to be 1.08%, whereas with the third-generation, it came out to be 0.31% in our study.
| Donors’ characteristics | Reactive group frequency (n=59) (%) | Negative group frequency (n=59) (%) | P-value |
|---|---|---|---|
| Age (years) | |||
| 18–25 | 22 (37.3) | 23 (39) | 0.712 |
| 26–30 | 13 (22) | 19 (32.2) | |
| 31–35 | 11 (18.6) | 9 (15.3) | |
| 36–40 | 3 (5.1) | 3 (5.1) | |
| 41–45 | 6 (10.2) | 3 (5.1) | |
| 46–50 | 4 (6.8) | 2 (3.4) | |
| Occupation | |||
| Professional | 21 (35.6) | 15 (25.4) | 0.793 |
| Arithmetic Skill Jobs | 10 (16.9) | 11 (18.6) | |
| Skilled worker | 1 (1.7) | 4 (6.8) | |
| Semi-skilled workers | 4 (6.8) | 6 (10.21) | |
| Unskilled worker | 4 (6.8) | 3 (5.1) | |
| Unemployed | 19 (32.2) | 20 (33.9) | |
| Education | |||
| School level | 8 (13.6) | 15 (25.4) | 0.534 |
| Diploma | 16 (27.1) | 16 (27.1) | |
| Bachelor level | 28 (47.5) | 24 (40.7) | |
| Master’s degree | 7 (11.9) | 4 (6.8) | |
| Type of donor | |||
| Replacement | 57 (96.4) | 55 (93.2) | 0.679 |
| Voluntary | 2 (3.4) | 4 (6.8) | |
| Blood group-ABO | |||
| A | 13 (22) | 12 (20.3) | 0.971 |
| B | 25 (42.4) | 24 (40.7) | |
| AB | 1 (1.7) | 1 (1.7) | |
| O | 20 (33.9) | 22 (37.1) | |
| Blood group-Rh | |||
| Positive | 55 (93.2) | 56 (94.9) | 1 |
| Negative | 4 (6.2) | 3 (5.1) | |
ELISA: Enzyme-linked immunosorbent assay
| Test | Frequency | Percentage |
|---|---|---|
| Fourth-generation ELISA | ||
| Positive | 59 | 50 |
| Negative | 59 | 50 |
| Third-generation ELISA | ||
| Positive | 17 | 14.4 |
| Negative | 101 | 85.6 |
| ELFA/Mini-Vidas (Antigen/Ab) | ||
| Positive | 14 | 11.8 |
| Negative | 104 | 88.2 |
| PCR | ||
| Positive | 13 | 11 |
| Negative | 105 | 89 |
| Rapid test | ||
| Positive | 14 | 11.8 |
| Negative | 104 | 88.2 |
HIV: Human immunodeficiency virus, ELISA: Enzyme-linked immunosorbent assay, ELFA: Enzyme-linked fluorescence assay, PCR: Polymerase chain reaction
The Table 1 compares different donor variables between fourth-generation ELISA reactive and negative groups. It showed no statistically significant difference between the two groups in terms of age, ABO and Rh blood group, occupation, education level, and residence.
We used Cohen’s Kappa coefficient[5] [see methodology] to determine the level of agreement between third- and fourth-generation HIV ELISA. In our study, we found κ to be 0.186. This demonstrates a slight level of agreement between third- and fourth-generation ELISA for the detection of HIV in blood donors.
The agreement between 4th-generation ELISA and PCR was tested using Kappa coefficients [Table 3]. We got the Kappa value to be 0.22, which is a fair level of agreement. The agreement between third-generation ELISA and PCR was also tested, and we got the Kappa value to be 0.848, which is a near-perfect agreement.
| PCR positive | PCR negative | Total | κ | P-value | |
|---|---|---|---|---|---|
| Fourth-generation ELISA positive | 13 | 46 | 59 | 0.22 | <0.001 |
| Fourth-generation ELISA negative | 0 | 59 | 59 | ||
| Third-generation ELISA positive | 13 | 4 | 17 | 0.848 | <0.001 |
| Third-generation ELISA negative | 0 | 101 | 101 | ||
| Total | 13 | 105 | 118 |
ELISA: Enzyme-linked immunosorbent assay, PCR: Polymerase chain reaction
The agreement between the fourth-generation ELISA and the rapid test used the same Cohen’s Kappa coefficient. We got a kappa value of 0.237, which is a fair level of agreement. In the agreement between the third-generation ELISA and the rapid test, we got a kappa value of 0.748, which is a substantial agreement.
DISCUSSION
TTI is still a major issue that contributes to significant morbidity and mortality worldwide. The global distribution of TTIs varies by region of the world and even between nearby nations. In India also, there is a considerable variation in HIV seroprevalence among different regions. The estimated HIV seroprevalence rate in India in 2021 was 0.21%, whereas in Puducherry, it was 0.32%.[6] A systemic review and meta-analysis by Bajpai et al. found that the blood donors from Western India had the most significant seroprevalence (0.433%), followed by those from Southern India (0.349%), Eastern India (0.34%), and Northern India (0.276%).[2] A study conducted by Cherukat et al. observed that the seroprevalence of HIV was 0.30% in blood donors in Puducherry.[7] In the present study, the prevalence of HIV by third-generation was 0.31% (3.13/1000 donations), whereas by fourth-generation was 1.08% (10.8/1000 donations). The prevalence of HIV by third-generation in our study is comparable to the findings by Bajpai et al.[2] They found that the overall pooled HIV prevalence rate among Indian blood donors was 0.32%.[2] In contrast, it is slightly higher than the findings of Makroo et al.[8] The prevalence of HIV by fourth generation in our study was 1.08%, which is higher than most other Indian studies. It could be due to the fact that fourth generation is more sensitive than third-generation ELISA. In our study, 59 samples were reactive by the fourth generation versus 17 samples by the third generation, leading to increased seroprevalence in fourth-generation ELISA. The other reason for increased positivity in fourth-generation could be false reactive/higher sensitivity. In contrast, a study conducted by Malhotra et al. found that the seroprevalence of HIV by third-generation ELISA was 0.12% and 0.36% by fourth-generation ELISA (P > 0.05).[9] The difference in the seroprevalence could be due to the fact that they did not use NAT to confirm the results of fourth-generation ELISA. A study conducted in southwest Nigeria in 2009 by Buseri et al. showed that the overall seroprevalence of HIV was 3.1%, which was high compared to most Indian studies. HIV seropositivity was present in more men (n = 36; 81.8%) than women (n = 8; 18.2%).[10] The reasons behind these variations in seroprevalence may be due to the endemicity of the infection, genotype of HIV, window period donations, type of donors (voluntary/replacement), donor selection process, differences in the screening strategies, and also differences in the tests that are used for testing samples. It is widely known that different test generations used for screening given blood have different sensitivity and specificity levels, which affect the total reactivity rate. Therefore, testing donated blood for TTIs is essential to ensure blood safety, particularly if the blood donor donated during the window time phase.
The agreement between third- and fourth-generation HIV ELISA for the detection of HIV in blood donors was studied using Cohen’s Kappa coefficient (K). The value of K ranges from 0 to 1, where o indicates no agreement, versus 1 means perfect agreement. We got a K value of 0.186, which suggests slight agreement. Out of 59 reactive samples by fourth-generation ELISA, only 17 were reactive in third-generation ELISA. Out of these, 13 came to be positive in PCR. Only one sample was negative in the third generation but was positive in the fourth generation, rapid test and ELFA test. This was negative in PCR, which could be due to some inhibitors in the test sample that reacted with the PCR reagent. Another explanation is that viral RNA is not found in the latent phase of infections, but antibody is still present. However, we were not able to repeat PCR due to insufficient samples. The reason for slight agreement between third- and fourth-generation HIV ELISA could be due to a more significant number of false positives by fourth-generation ELISA. Fourth-generation ELISA being more sensitive than third-generation ELISA has more chance of giving false positivity. This has been shown in some studies.[11] A study conducted by Malhotra et al. also concluded that the relatively high false reactive rate of the fourth-generation ELISA is another drawback.[9] This is primarily due to high concentrations of immunoglobulin G (IgG) in the blood. Some of these IgG molecules adhere to the microwell surfaces, leading to a false-positive result. Other causes for false positivity could be due to acute recent infections caused by other agents, IgG or immunoglobulin M that are cross-reactive, vaccinations such as influenza, and polyreactive antibodies.[11] This might have led to a significant number of HIV cases by fourth-generation ELISA than third-generation ELISA, leading to a slight agreement between them.
The agreement between fourth-generation HIV ELISA and PCR was also studied using Kappa statistics, which turned out to be 0.22, which indicates a fair level of agreement. The fair level of agreement between fourth-generation HIV ELISA and PCR could be due to false-positive cases picked up by fourth-generation ELISA, which were negative in PCR. This led to the maximum number of negative reactive samples in the PCR test. However, no fourth-generation ELISA negative turned was positive in PCR, that is, there were no false-negative results. This is similar to the findings of Mathur et al.[12] They also concluded that no sample tested positive for HIV in NAT after being subjected to fourth-generation ELISA negative tests, that is, HIV NAT yield was zero.[12] Similar findings were found out by Kumar et al.[13] in their study conducted in a total of 1599 blood donors in Delhi in 2018. Four reactive samples were detected by fourth-generation ELISA, of which two samples had marginal positivity. Although all four samples were negative by the rapid test, they were all positive by PCR. They concluded that the sensitivity of fourth-generation ELISA was comparable to PCR while rapid was significantly inferior to both fourth-generation ELISA and PCR.[13]
As per National AIDS Control Society strategy, the positive result of the first screening test should either be taken as such or confirmed by another one or two screening tests. The first screening test should be highly sensitive, whereas the second and third should have high specificity. For transfusion and transplantation purposes, the result of 1st test is enough to accept or discard the unit. The positivity in 1st test could be true positive or false positive that cannot be distinguished unless a supplemental or confirmatory test is used. Onetime positive donors are deferred permanently from blood donations. In India, there is no provision for donor re-entry which might have led to the increased number of permanent deferral of donors. If one more test could be applied after the initial screening test, unnecessary discards due to false positivity could have been prevented. If any discrepancies exist between 1st and 2nd tests, such donors can be followed up a certain time and re-tested, and, if negative, they can be put into the donor pool for future donations. This will decrease the unnecessary discards as well as can positively impact donors who were deferred due to false-positive results in a single test.
CONCLUSION
Our study showed a fair level of agreement between third- and fourth-generation ELISA for the detection of HIV in blood donors. Although fourth-generation HIV ELISA has high sensitivity, false positives are more in comparison to third-generation ELISA. Due to this, the fourth-generation ELISA kit may give a greater number of discards. It’s high time for India to allow donor re-entry if a repeat test after some period is negative. The present study will add to the knowledge and better understanding of testing of HIV in blood donors to the existing literature. Further studies will be required to verify our findings and to know more about HIV in blood donors.
Ethical approval
The research/study approved by the Institutional Review Board at Jawaharlal Institute of Postgraduate Medical Education and Research, number JIP/IEC/2021/0117, dated 12th June, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: This work was supported by an intramural research fund from the Jawaharlal Institute Postgraduate Medical Education and Research (JIPMER), Pondicherry, India.
References
- HTLV-III/LAV infection in nine children infected by a single plasma donor: Clinical outcome and recognition patterns of viral proteins. J Infect Dis. 1986;154:171-4.
- [CrossRef] [PubMed] [Google Scholar]
- Seroprevalence and trend of human immunodeficiency virus infection among Indian blood donors: A systematic review and meta-analysis. Glob J Transfus Med. 2020;5:129-34.
- [CrossRef] [Google Scholar]
- A comparative study for screening human immunodeficiency virus 1/human immunodeficiency virus 2 with third-generation and fourth-generation human immunodeficiency virus ELISA kits in donors from a tertiary care hospital in Northeast India. Curr Med Issues. 2019;17:108.
- [CrossRef] [Google Scholar]
- Fourth-generation enzyme-linked immunosorbent assay for the simultaneous detection of human immunodeficiency virus antigen and antibody. J Clin Microbiol. 2001;39:2518-24.
- [CrossRef] [PubMed] [Google Scholar]
- Interrater reliability: The kappa statistic. Biochem Med (Zagreb). 2012;22:276-82.
- [CrossRef] [PubMed] [Google Scholar]
- Surveillance - National AIDS Control Organization - MoHFW - GoI. Available from: https://www.naco.gov.in/surveillanceepidemiology-0 [Last accessed on: 2023 Jul 25]
- [Google Scholar]
- Seroprevalence of transfusion-transmitted infections among blood donors in a tertiary care hospital in Puducherry. J Prim Care Spec. 2022;3:8.
- [CrossRef] [Google Scholar]
- Seroprevalence of infectious markers and their trends in blood donors in a hospital based blood bank in North India. Indian J Med Res. 2015;142:317.
- [CrossRef] [PubMed] [Google Scholar]
- Seroprevalence of human immunodeficiency virus in north Indian blood donors using third and fourth generation Enzyme linked immunosorbent assay. Asian J Transfus Sci. 2013;7:125-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sero-epidemiology of transfusion-transmissible infectious diseases among blood donors in Osogbo, south-west Nigeria. Blood Transfus. 2009;7:293-9.
- [Google Scholar]
- False positive viral marker results in blood donors and their unintended consequences. Vox Sang. 2018;113:530-9.
- [CrossRef] [PubMed] [Google Scholar]
- Nucleic acid test versus enzyme linked section immunosorbent assay for screening of human immunodeficiency virus in donated blood units: A comparative study at a blood centre in Western Rajasthan, India. J Clin Diagn Res. 2022;16:EC16-21.
- [CrossRef] [Google Scholar]
- Comparison of HIV fourth generation rapid test with HIV fourth generation ELISA for screening voluntary blood donors. Indian J Appl Res. 2018;8:8-11.
- [Google Scholar]







