Translate this page into:
Low dose long-acting factor VIII prophylaxis in pediatric and young adult patients with hemophilia A: Short-term single-center experience from a developing country
-
Received: ,
Accepted: ,
How to cite this article: Verma SP, Tripathi AK, Sharma GS, Kumar N, Kushwaha R. Low dose long-acting factor VIII prophylaxis in pediatric and young adult patients with hemophilia A: Short-term single-center experience from a developing country. J Hematol Allied Sci 2021;1:75-80.
Abstract
Objectives:
High dose factor prophylaxis in hemophilia has been proven to prevent joint bleeds in the western world effectively. We look for a cost-effective and feasible way for Indian patients to reduce the dose and frequency of factor infusion. Data on prophylaxis with a low dose, long-acting factor infusion twice a week dosing schedule is limited. The purpose was to study the efficacy and safety of long-acting factor VIII (Eloctate) for secondary/ tertiary prophylaxis in pediatric and young adult patients with moderate and severe hemophilia A.
Materials and Methods:
Thirty-eight patients with moderate and severe hemophilia A with an age range from 1 to 25 years were included in the study. During the initial 4 months, they received therapeutic doses of ELOCTATE (Factor VIII with Fc Fusion Protein) on an episodic basis after a clinical bleed. In the next 4 months, they received prophylactic intravenous ELOCTATE at the dose of 20 units/kg body weight twice a week. Annual bleeding rates (ABR), school absenteeism, emergency visits, joint scores, and adverse events were compared during both periods.
Results:
The total number of joint bleeds during the episodic treatment and prophylaxis period was 608 and 67, respectively. ABR was 47.9 during the episodic treatment period and 5.3 during prophylaxis showing an 88.9% reduction in joint bleeds. School/college absenteeism and emergency visits were significantly reduced during prophylaxis. No significant adverse events were noted during prophylaxis.
Conclusion:
Low dose, twice a week, and long-acting recombinant factor VIII-Fc (Eloctate) prophylaxis can be a reasonable options for patients with hemophilia A in developing countries.
Keywords
Annualized bleeding rates
Eloctate
Hemophilia A
Long acting recombinant Factor VIII-Fc Fusion product
Tertiary prophylaxis
INTRODUCTION
Hemophilia is an X-linked inherited coagulation disorder.[1] Almost 80% of bleeds in hemophilia are limited to joints. Joint bleeds lead to long-term comorbidities in hemophilia, including chronic synovitis, crippling arthropathies, deformities, and chronic pain.[2] The life of a hemophiliac is miserable if factor concentrates are not available. Joint bleeds in hemophilia can be managed by two strategies, that is, episodic treatment or factor prophylaxis.
Factor prophylaxis is the standard of care for children and young adults in developed countries.[3] Usually, this is high dose factor prophylaxis using factor concentrate in doses of 25–40 units/kg of body weight.[4,5] Indian patients account for 1/3rd of the total hemophilia population in the world. In India, most of the states get factor concentrate through state governments for episodic treatment, although many centers such as army hospitals, ESI hospitals, and some state hospitals are practicing regular replacement therapy (prophylaxis). Episodic treatment, although it controls bleeding, cannot prevent the ongoing joint damage completely. Countries with cost constraints are continuously looking for effective and feasible prophylaxis methods in terms of reduced dose and frequency of factor infusion. Data on low dose factor prophylaxis are available from China and India.[6-8] These studies were carried out using plasma-derived very low dose factor VIII concentrate (10 U/kg body weight twice a week), which has a short half-life of 10–12 h.
Prophylaxis with short t1/2 factor concentrate, leads to frequent venous access and treatment burden on hemophilia patients and their parents. Due to this, in long run, compliance is reduced and outcomes are poor. Many long-acting factor concentrates have flourished in the past 10 years, including rFVIII-Fc fusion protein (Eloctate). Data on the efficacy of low dose extended t1/2 factor VIII prophylaxis on a twice-weekly dosing schedule are limited.[9] A very recent study from the western part of India conducted by Gulshan et al. has shown promising results with Eloctate at doses of 10 units/kg body weight, 2 times in a week.[10] In this study, Eloctate was given as secondary/tertiary prophylaxis and resulted in an 85% reduction in joint bleeds.
Eloctate is an anti-hemophilic factor prepared by fusion of Recombinant factor VIII with the Fc portion of Immunoglobulin. Fusion to Fc fragment of Immunoglobulin increases half-life of factor VIII to 19 h, almost 1.5 times the t1/2 of standard plasma-derived or recombinant factor VIII.[11] Long half-life makes Eloctate a very suitable option for prophylaxis. The recommended dose of Eloctate in the United States is 50 IU/Kg body weight every 4th day, and the regimen may be adjusted in the range of 25–65 IU/Kg every 3–5 days based on the response.[12] This schedule involves very high costs and practically remains a non-affordable treatment option in developing countries like India. We used lower doses of eloctate, that is, 20 units/Kg twice a week (Tuesday and Friday), which is almost half the dose used in the US. World Federation of Hemophilia (WFH) provided Eloctate on humanitarian ground.
Aims and objectives
Primary objectives
The primary objective was to study annualized bleeding rate (ABR) and rFVIII-Fc fusion protein (Eloctate) safety during episodic treatment and factor prophylaxis.
Secondary objectives
The secondary objectives were to study days of school absenteeism, number of emergency visits, Inhibitor development, Hemophilia joint health score (HJHS) 2.1 score, Functional independence score in hemophilia (FISH), and adverse events during episodic treatment and prophylaxis.
MATERIALS AND METHODS
The study was conducted in the Department of Clinical Hematology, King George’s Medical University, Lucknow, Uttar Pradesh. The Institute Ethics committee approved this study. Informed consent was taken from the patients, parents, or legally authorized relatives in their regional language. The study was conducted in compliance with the ethical standards as well as with the Helsinki Declaration. It was a prospective interventional study. Thirty-eight patients with moderate and severe hemophilia A with an age range of 1–25 years and fulfilling the eligibility criteria for the study were selected. These patients routinely attended the hemophilia comprehensive care (HCC) center in the department for episodic treatment and physiotherapy. During the study, patients received episodic treatment for 4 months, followed by factor prophylaxis for the next 4 months.
During episodic treatment, patients received therapeutic doses of long-acting factor VIII (Eloctate) once a day based on the bleed site according to the WFH guidelines. In the next 4 months, they received prophylactic eloctate, given intravenously through a peripheral vein at the dose of 20 unit/kg body weight on twice a week (Tuesday and Friday) schedule at our HCC center by trained hemophilia care providers (nurse and physiotherapist). Prophylaxis intended to provide secondary or tertiary prophylaxis as most of these patients already had established joint problems. Following were the inclusion and exclusion criteria.
Inclusion criteria
Patients with severe and moderate hemophilia A within the age group of 1–25 years and without inhibitors were included in the study.
Exclusion criteria
The following criteria were excluded from the study:
Patients of Hemophilia A with Inhibitors
Patients not providing informed written consent
Patients with other significant orthopedic anomalies
Patients with hepatic dysfunction or chronic liver disease.
Data were collected weekly and entered inpatient records during the entire period of 8 months. During episodic treatment, it was collected telephonically once a week and by physical presence once a month or when the patient came for episodic factor infusion. Number of bleeds, sites of bleed, the dose of a factor given, number of emergency visits, and days of school absenteeism were noted for all eligible patients during both phases. HJHS 2.1 and FISH score assessments were done both at the end of episodic treatment and prophylaxis period. Inhibitor testing using modified Bethesda assay was done at baseline and the end of both study phases. Factor trough levels and recovery levels were done once during the mid of the prophylaxis period. Prophylaxis was given on a fixed-dose basis without pharmacokinetic monitoring.
Statistics
The results were analyzed using descriptive statistics and making comparisons between the groups with respect to various parameters. Discrete (categorical) data were summarized as in proportions, and percentages (%) and quantitative data were summarized as Mean ± standard deviation (SD).
The nonparametric Wilcoxon signed-rank test was used for comparing two related matched samples to assess whether their population mean ranks differ. It is used as an alternative to the paired Student’s t-test in a case when the population cannot be assumed to be normally distributed.
The paired sample t-test was used to determine whether the mean difference between two paired sets of observations is zero. The Gaussian test is used to determine whether the proportion between the two sets of observations is equal.
A two-tailed P < 0.05 was considered statistically significant. All the statistical analysis was done using IBM-SPSS-16 statistical software.
RESULTS
A total of 38 patients participated in this study, and 33 (87%) were having severe hemophilia A. Mean age of the patients at study participation was 13.39 ± 6.32 years (Range 2–25 years). The mean age of patients at the time of diagnosis of hemophilia was 32.29 ± 50.70 months (range 1–228 months).
The total number of joint bleeds during the episodic treatment and prophylaxis period was 608 and 67, respectively. The total number of bleeds during the episodic treatment and prophylaxis period was 653 and 74, respectively. The annualized joint bleed rate was 47.9 during the episodic treatment period and 5.3 during prophylaxis. Total factor consumption during episodic treatment was approximately 390,000 units compared to 798,000 units during prophylaxis which indicated almost double amount of factor required during prophylaxis. A significant reduction of 88.7%, 89%, and 88.9% was seen in the total number of bleeds, the number of joint bleeds, and annualized joint bleeding rate during prophylaxis. [Table 1] shows the total number of joint bleeds, muscle bleeds, ABR, and infusion-related events during this period.
| Bleeding Summary | Episodic treatment period (n=38) | Prophylaxis period (n=38) | % Reduction (P-value) |
|---|---|---|---|
| Total number of bleeds | 653 | 74 | 88.7 (<0.05) |
| Total joint bleeds | 608 | 67 | 89.0 (<0.05) |
| Muscle bleeds | 36 | 4 | 88.9 (<0.05) |
| Annualized joint bleed rate | 47.9 | 5.3 | 88.9 (<0.05) |
| Infusion related events | 2 | 5 | (ns) |
[Table 2] shows the average number of individual joint bleeds per patient during episodic treatment and the prophylaxis period. Individual bleeds, including knee, elbow, wrist, ankle, psoas bleeds, and other muscle bleeds, were significantly reduced during factor prophylaxis. Knee joint bleed was the most common bleed, followed by elbow and ankle bleed. [Figure 1] shows a bar diagram showing different sites and frequency of bleeds during both phases of the study.
| Sites of Bleed | Episodic treatment period number of bleeds (mean±SD) | Prophylaxis period number of bleeds (mean±SD) | % Change in mean | P-value |
|---|---|---|---|---|
| Knee | 7.14±6.18 | 1.03±1.36 | 85.61 | <0.001 |
| Elbow | 5.21±4.77 | 0.58±1.00 | 88.89 | <0.001 |
| Ankle | 3.26±4.40 | 0.13±0.41 | 95.97 | <0.001 |
| Wrist | 0.00±0.00 | 0.00±0.00 | NA | 1.0 |
| Psoas muscle | 0.34±0.88 | 0.00±0.00 | 100 | 0.016 |
| Other Joints | 0.68±1.32 | 0.21±0.62 | 69.23 | 0.034 |
| Other Muscles | 0.95±1.82 | 0.11±0.39 | 88.89 | 0.009 |
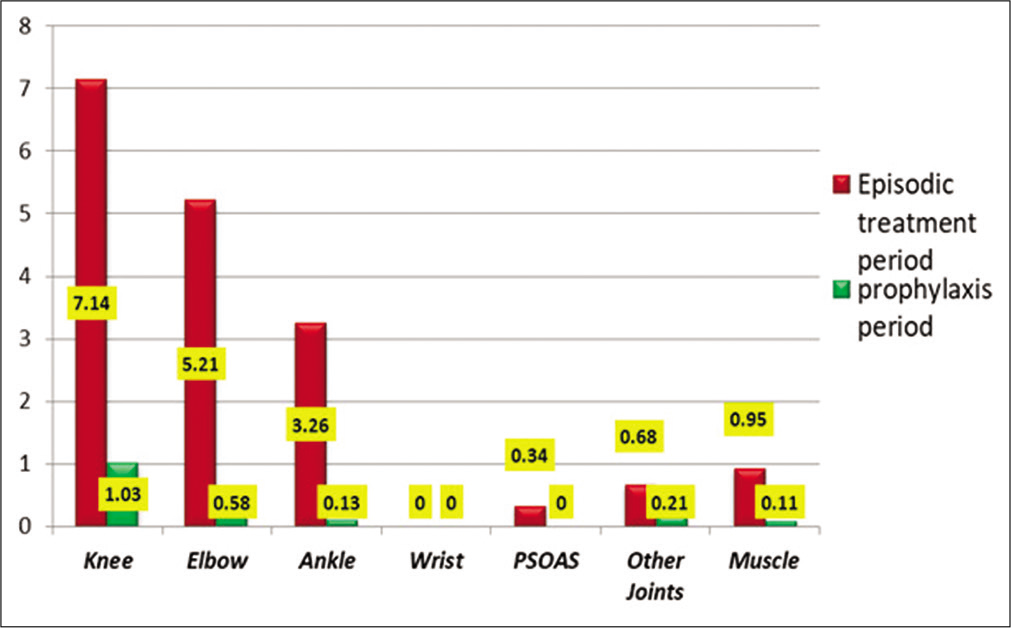
- Average number of bleeds at various sites during the episodic treatment period and the prophylaxis period.
[Table 3] shows the details of five patients who were diagnosed as moderate hemophiliacs at baseline. The bleeding phenotype of these patients was similar to severe hemophilia. Total number of bleeds during episodic treatment was 90, which reduced to 14 during prophylaxis with eloctate. There was 84.5% reduction in bleeding episodes similar to severe hemophilia patients.
| Patients | Age (years) | Factor level at diagnosis (%) | Bleeds during episodic treatment | Bleeds during prophylaxis |
|---|---|---|---|---|
| 1 | 8 | 2 | 15 | 3 |
| 2 | 20 | 2.5 | 18 | 2 |
| 3 | 10 | <2 | 8 | 5 |
| 4 | 16 | 1.5 | 22 | 3 |
| 5 | 13 | 1.3 | 27 | 1 |
HJHS 2.1 and FISH were calculated at the end of episodic treatment and prophylaxis. Both HJHS 2.1and FISH scores showed improvement during the prophylaxis period compared to the episodic treatment period. School/college absenteeism was 3.1 days/month and 0.84 days/month during episodic treatment and prophylaxis, respectively. Average emergency room visits were 11.9 during episodic treatment and 2.3 during prophylaxis. Emergency visits were significantly more during episodic treatment. [Table 4] below shows the HJHS 2.1, FISH, school absenteeism, and emergency visits during the episodic treatment period and prophylaxis.
| Test | Episodic treatment period (mean±SD) | Prophylaxis period (mean±SD) | % Change in mean | P-value |
|---|---|---|---|---|
| HJHS (n=38) | 13.53±9.13 | 8.34±6.84 | 38.33 | <0.001 |
| Functional independent score in hemophilia (n=36) | 26.76±4.92 | 29.42±3.24 | 9.93 | <0.001 |
| Days of school absenteeism (n=36) | 12.57±10.22 | 3.27±5.71 | 73.98 | <0.001 |
| No. of emergency room visits (n=38) | 11.92±5.98 | 2.37±3.27 | 80.13 | <0.001 |
HJHS: Hemophilia joint health score, FISH: Functional independence score in hemophilia
None of the patients developed inhibitors during episodic treatment or prophylaxis. Five patients had mild superficial thrombophlebitis during prophylaxis compared to two patients during episodic treatment. Thrombophlebitis improved with supportive measures in all patients.
Trough and recovery levels were studied during mid of prophylaxis period. Trough level was done before factor infusion, and recovery level was estimated 1-h post-infusion. The trough status was found to be 2.42 ± 1.56%, and the factor recovery level was 14.06 ± 9.52%.
DISCUSSION
Factor prophylaxis is the standard of care for hemophilia worldwide. Many landmark studies have proven the role of factor prophylaxis.[4,5,12,13] Usually, these studies are aimed at primary prophylaxis because that is the only way to achieve pristine joints. These studies used moderate to high doses of plasma-derived/recombinant factor products 3 times a week for hemophilia A and 2 times a week for hemophilia B. Malmo protocol and Utrecht Protocols are the well-known protocols for prophylaxis in the western world.[12-15] Developing countries looked for low dose or very low dose factor prophylaxis protocols 2 times a week for their hemophilia patients with >85% reduction in joint bleeds, although complete prevention of bleeding could not be achieved.[6-9]
Current prophylaxis protocols are very effective but still cannot prevent joint damage completely. Patients receiving regular prophylaxis still have breakthrough bleeds and develop joint arthropathies maybe a decade or two later. The current need in hemophilia is to provide factor concentrates based on the pharmacokinetic profile of individual patients, the patient’s physical needs, and the use of long-acting factor concentrates for prophylaxis.[16,17] Factor prophylaxis has been proven to be effective in adolescents and adults as well.[18,19] Standard t1/2 factors need to be infused 3 times a week, which becomes a considerable treatment burden in the long term for all patients.
At present, three long-acting factor concentrates are available, including recombinant factor VIII Fc fusion protein (rFVIII-Fc), recombinant factor VIII pegylated (rFVIII-PEG), and FVIII single chain (FVIII-sc).[11] All are proven to be equally efficacious considering the bleeding rates. Factor dose utilization may be slightly lower with the FVIII-sc product. The significant benefit of these products is to decrease the frequency to 2 times a week or maybe lower, decreasing the treatment burden on the patients.[20-23] This is more relevant in the Indian scenario where patients have to travel long distances to reach a hospital, and they are still not confident enough to get venous access for home-based factor prophylaxis.
In this study, we demonstrated that low to medium dose (20 IU/Kg BW twice a week) long-acting factor VIII-Fc concentrate given as secondary/tertiary prophylaxis can result in 88.9%, 89%, and 88.9% reduction in total number of bleeds, total number of joint bleeds, and ABR, respectively, in pediatric and young adult patients with hemophilia A. A similar study by Gulshan et al. in the pediatric population (median age 6.5 years) using even lower doses of Eloctate at 10 IU/kg BW twice a week has shown an 85.7% reduction in annualized bleed rates.[10] This finding supports that the lower doses of Eloctate can be quite effective where the state’s economic condition cannot afford high dose factor prophylaxis. Our study’s higher median ABR (5.3 vs. 2.2) is probably due to the much older population in our study receiving tertiary prophylaxis.
A-LONG and Kids A- LONG are the two-phase III studies which analyzed the role of rFVIII-Fc concentrate in severe hemophilia A patients.[24,25] While A-long included patients aged >12 years, the Kids A-LONG study was for children <12. A-LONG analyzed the role of the rFVIII-Fc product in a three-arm study, including individualized prophylaxis (arm-1), weekly prophylaxis (arm-2), and episodic treatment (arm-3). ABRs reduced by 92% in arm1, and 76% in arm-2 compared to arm-3. There was no development of new inhibitors in the study population. A weekly higher median dose of factor VIII Fc (77.7 Units/kg compared to 40 Units/kg in our study) and monitored pharmacokinetic dosing explains the lower ABR compared to our study. Kids A-LONG annual bleed rate was 1.96 with 0.0% spontaneous bleeds. There was a 93% reduction in bleeding episodes, and 43% of patients did not have any bleeding. Our study has shown similar results in reducing the bleeding frequency but higher annual bleed rate probably due to the lower factor dose (40 IU/kg vs. 88.11 IU/Kg) and higher age of hemophilia patients.
Poor and severely damaged joints can explain higher annual bleed rates (5.3 vs. 1.96 in Kids A-LONG and 1.6 in A-LONG) in our study in our patients. These patients do not get routine prophylaxis in India, so they tend to bleed more with increasing age because of more synovial inflammation and friability. This finding is evident by comparing the episodic treatment groups of both studies. Our patients had an ABR of 47 compared to 33.4 in the A-LONG study. Another reason may be that almost half the weekly prophylactic dose used in our study compared to Kids A-LONG and A-LONG studies.
Joint scores including HJHS 2.1 and FISH showed improvement during the prophylaxis compared to the observation period. Our patients had poorer joint scores compared to other studies on prophylaxis because of the higher age group. Another study using intermediate and high dose prophylaxis has also shown that in patients who get regular prophylaxis, they have HJHS scores below 10.[26]
CONCLUSION
Long-acting factor VIII formulation (rFVIII-Fc, Eloctate) has a promising role in prophylaxis for hemophilia patients. Low dose, twice a week prophylaxis with this agent can be a reasonable option for patients with hemophilia A in developing countries. It significantly reduces joint bleeds, overall bleeds, school absenteeism, emergency visits, and improves joint scores without risk of inhibitor formation and any significant adverse events.
Acknowledgments
The authors are grateful to the participants enrolled in the study, staff members of the department for extending help in hematological services, and especially thank to the World Federation Hemophilia, for providing Eloctate on humanitarian ground, without which it would not have been possible to carry out the study.
Authors contribution
SPV and AKT planned the study and reviewed the literature. GSS and RK helped in conduction of study including joint assessments and laboratoty workup. NK helped in laboratory work up as well as proofreading of the write up.
Availability of data for transparency
Yes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Recombinant FVIII-Fc (Eloctate) was provided by WFH Humanitarian Aid, no other financial support received from anywhere.
Conflicts of interest
There are no conflicts of interest.
References
- Establishing the prevalence and prevalence at birth of hemophilia in males: A meta-analytic approach using national registries. Ann Intern Med. 2019;171:540-6.
- [CrossRef] [PubMed] [Google Scholar]
- The past and future of haemophilia: Diagnosis, treatment and its complications. Lancet. 2016;388:187-97.
- [CrossRef] [Google Scholar]
- Hemophilia therapy: The future has begun. Hematologica. 2020;105:545-53.
- [CrossRef] [PubMed] [Google Scholar]
- Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535-44.
- [CrossRef] [PubMed] [Google Scholar]
- The ESPRIT Study Group. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study) J Thromb Haemost. 2011;9:700-10.
- [CrossRef] [PubMed] [Google Scholar]
- Low dose secondary prophylaxis reduces joint bleeding in severe and moderate haemophilic children: A pilot study in China. Haemophilia. 2011;17:70-4.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized study of very low dose factor VIII prophylaxis in severe haemophilia-a success story from a resource limited country. Haemophilia. 2016;22:342-8.
- [CrossRef] [PubMed] [Google Scholar]
- Low dose prophylaxis for children with haemophilia in a resource-limited setting in south India-a clinical audit report. Hemophilia. 2017;23:e382-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of tertiary prophylaxis with low-dose factor VIII in quality of life in adult patients with severe hemophilia A. J Appl Hematol. 2019;10:88-93.
- [CrossRef] [Google Scholar]
- Is low dose a new dose to initiate hemophilia A prophylaxis? -A systematic study in Eastern India. Indian J Pediatr. 2020;87:345-52.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing factor usage and bleed rate in US Hemophilia A patients receiving prophylaxis with 3different long acting recombinant factor VIII products. J Manag Care Spec Pharm. 2020;26:504-12.
- [CrossRef] [PubMed] [Google Scholar]
- Twenty-five years' experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25-32.
- [CrossRef] [PubMed] [Google Scholar]
- Haemophilia prophylaxis in young patients-a long-term follow-up. J Intern Med. 1997;241:395-400.
- [CrossRef] [PubMed] [Google Scholar]
- Prophylactic versus on-demand treatment strategies for severe haemophilia: A comparison of costs and long-term outcome. Haemophilia. 2002;8:745-52.
- [CrossRef] [PubMed] [Google Scholar]
- Prophylactic treatment for severe haemophilia: Comparison of an intermediate-dose to a high-dose regimen. Haemophilia. 2002;8:753-60.
- [CrossRef] [PubMed] [Google Scholar]
- Improved cost-effectiveness by pharmacokinetic dosing of factor VIII in prophylactic treatment of haemophilia A. Haemphilia. 1997;3:96-101.
- [CrossRef] [PubMed] [Google Scholar]
- Multidose pharmacokinetics of factor IX: Implications for dosing in prophylaxis. Haemophilia. 1998;4:83-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prophylaxis in adults with haemophilia. Haemophilia. 2007;13(Suppl 2):10-5.
- [CrossRef] [PubMed] [Google Scholar]
- Should prophylaxis be used in adolescent and adult patients with severe haemophilia? An European survey of practice and outcome data. Haemophilia. 2007;13:473-9.
- [CrossRef] [PubMed] [Google Scholar]
- Fc-fusion technology and recombinant FVIII and FIX in the management of the hemophilias. Drug Des Dev Ther. 2014;8:365-71.
- [CrossRef] [PubMed] [Google Scholar]
- Changing paradigm of prophylaxis with long acting factor concentrate. Hemophilia. 2014;20(Suppl 4):99-105.
- [CrossRef] [PubMed] [Google Scholar]
- Optimal treatment strategies for haemophilia: Achievements and limitations of current prophylaxis regimens. Blood. 2015;25:2038-44.
- [CrossRef] [PubMed] [Google Scholar]
- Predicting the outcomes of using longer-acting prophylactic factor VIII to treat people with severe hemophilia A: A hypothetical decision analysis. J Thromb Haemost. 2015;14:2141-7.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123:317-25.
- [CrossRef] [PubMed] [Google Scholar]
- Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost. 2015;13:967-77.
- [CrossRef] [PubMed] [Google Scholar]
- Intermediate-dose versus high-dose prophylaxis for severe hemophilia: Comparing outcome and costs since the 1970s. Blood. 2013;15:1129-36.
- [CrossRef] [PubMed] [Google Scholar]






