Translate this page into:
Malaria parasite density relationship with spleen size and fetal hemoglobin in Nigerian children with homozygous sickle cell disease
*Corresponding author: Abubakar Garba Farouk, Department of Paediatrics, University of Maiduguri, Maiduguri, Nigeria. farouk649@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Farouk AG, Ibrahim HU, Yusuf HM, Bukar L, Idrisa JA, Usman AU. Malaria parasite density relationship with spleen size and fetal hemoglobin in Nigerian children with homozygous sickle cell disease. J Hematol Allied Sci. 2024;4:23-31. doi: 10.25259/JHAS_32_2023.
Abstract
Objectives:
This study aimed to determine the malaria parasite density in children with homozygous sickle cell disease and compare it with the splenic size and fetal Hb (HbF).
Material and Methods:
In this cross-sectional study, we determined the malaria parasite density of children with sickle cell anemia (SCA) in a steady state aged 2–15 years and compared it with aged-matched HbAA controls. The HbF levels of children with SCA in a steady state were also determined by the Betke alkali denaturation method. The spleen size was determined both clinically and sonographically. The relationship between malaria parasite density, spleen size, and HbF was also determined.
Results:
Subjects with HbSS 440 (360–600) had a higher parasite density than HbAA controls 200 (200–360). This was statistically significant (U-stat = 1728, P = 0.001). Malaria parasite density progressively reduced with increasing age in HbSS subjects with normal spleen status, while the reverse is true in those with abnormal spleen status. The difference was statistically significant, especially among the 6–10 year age group (U-stat = 4.50, P = 0.001). Malaria parasite density appears to be lower in the age group 2–5 years with high HbF; however, the statistical test was not significant (H-test = 0.602, P = 0.740). The scatter plot shows a negative and positive correlation between the 2–5-year age group and the 6–10-year age group with malaria parasite density, respectively.
Conclusion:
Children with HbSS had a higher malaria parasite density than matched HbAA controls. Furthermore, a significant relationship exists between malaria parasite density and spleen size in children with SCA. HbF level was not significantly related to asymptomatic malaria parasitemia. It is recommended that the early introduction of hydroxyurea to the care of SCA children is necessary in their routine care to reduce morbidity and mortality from confounding infectious diseases, including malaria.
Keywords
Fetal hemoglobin
Homozygous
Malaria
Sickle cell
Spleen
INTRODUCTION
Homozygous sickle cell disease (HSCD) is the most common hereditary hemoglobin (Hb) disorder worldwide; the greatest burden of this disorder is in sub-Saharan Africa, where 75% of the 300,000 global births of affected children are found.[1] In all patients with inherited Hb disorder, Hb S (HbS) is the most predominant abnormal Hb component. In the carrier states, HbS or other abnormal Hb variants, such as HbC or thalassemia, coexist with normal adult Hb (HbA). The homozygous variant with HbSS, called sickle cell anemia (SCA), is the most severe form of these disorders and does not confer any survival advantage against severe clinical malaria due to Plasmodium falciparum comparable to that conferred by the heterozygous traits.[2] Sickle cell disease (SCD) and malaria are the worst hereditary and infectious tropical diseases, respectively, in the Northeast in particular and Nigeria in general. Unfortunately, the two prevalent disease states manifest with similar symptomatology of severe hemolytic anemia warranting blood transfusion, weakness, lethargy, body aches, as well as high morbidity and mortality rates.[3] HSCD arises from a point mutation in the β-globin gene, with the replacement of the acidic amino acid (glutamic acid) by a neutral amino acid residue (valine) at position six of the amino acid sequence of the β-globin chain, which forms HbS. In a hypoxic state, such as what is usually found in deep tissues and organs during infestation with malaria or other bacterial infections, the HbS get polymerized and aggregated into rigid tactoids ultimately distorting the biconcave shape of the normal red blood cells (RBCs) to form sickle shape which is the hallmark of the pathophysiology of SCA.[4] This resultant formation of rigid aggregated sickled RBCs in deep tissues and organs block the microvasculature, which ultimately causes intractable pains, cell death, and tissue or organ damage.[5] After about the age of 8 years, <10% of HSCD patients in the American continent have persistent splenomegaly.[6-8] Conversely, however, splenomegaly is found in much older age in sub-Saharan African patients with HSCD.[9] A landmark research by Esan reported a spleen rate of 15% in adult Nigerian HSCD patients.[10] There is ample evidence to show that this persistent splenomegaly in sub-Saharan African patients with SCA is believed to be associated with the pandemics of malaria in this region.[11,12] The heterozygous carriers (HbAS) of the sickle cell trait in a population produce a mixture of both normal erythrocytes having normal Hb and the sickle-shaped RBCs, especially in severe hypoxic conditions at the venous end of blood circulation, while the homozygous SCD (HbSS) in the population has a greater proportion of sickle cells in circulation. Despite the deleterious character of homozygotes, the sickle mutation exists in the human population since the heterozygotes have the selective advantage of resisting malaria parasitemia.[13] A landmark research by Allison linked heterozygotes to protection from malaria in Ugandans.[14] Consequently, in areas where malaria is prevalent, heterozygosity of sickle cells is more likely to survive and tends to increase the frequency of the sickle cell gene over the normal homozygotes (HbAA) as they are more susceptible to severe forms of malaria. Therefore, the sickle cell trait demonstrates a clear case of balanced polymorphism where the heterozygote’s advantage of protection from severe form of malaria coexists with disadvantageous homozygotes in a population of a malaria-endemic region.[15]
MATERIAL AND METHODS
Study design
This was a cross-sectional study that examined the relationship between malaria parasite density, splenic size, and fetal Hb (HbF).
Study site
This research was conducted at the Pediatric Hematology Clinic of the University of Maiduguri Teaching Hospital (UMTH) over 6 months from January to June. Located in the capital of the old Northeastern state of Nigeria and currently the capital of Borno State, UMTH is a Federal Government Tertiary health center serving as a referral hospital for the North-eastern of Nigeria. It also caters for the three neighboring countries of Cameroon, Chad, and the Niger Republic. The pediatric hematology clinic runs once a week with an average patient turnover of 40 per clinic day. It has over 700 registered patients. The patients are registered after confirmation of homozygous SS or heterozygous SC disease by Hb electrophoresis on cellulose acetate membranes. The UMTH has a bed capacity of 800 and an annual inpatient turnover of 120,000, while the pediatrics department of the hospital has a 96-bed capacity and an annual inpatient turnover of 14,000. Indigenous ethnic groups in Maiduguri are the Kanuris and Shuwa Arabs. However, there is also a sizable population of other ethnic groups and nationalities resulting from the internal displacement of people due to the Boko Haram insurgency. The Kanuris have one of the highest prevalence rates of sickle cell traits in Nigeria.[16] The pediatric hematology clinic functions as a regular follow-up unit, where non-critically ill patients and patients in a steady state are seen. Patients in crisis or ill are admitted to the Emergency Pediatric Unit. The services provided include counseling, blood transfusions, and provision of medicines, for example, antimalarial prophylaxis, folic acid, penicillin V, and analgesics. The clinic relies on Specialist and Resident doctors for the delivery of services. Disease-modifying drugs such as hydroxyurea are only routinely prescribed for eligible patients.
Study population
Children aged 5–15 years. Participants were recruited consecutively as they presented at the clinic after meeting the inclusion criteria.
Inclusion criteria
Children aged 5–15 years with homozygous SCD confirmed by Hb electrophoresis that were healthy in steady-state after consenting/assent. Children with HbAA on follow-up for minor ailments at pediatric outpatient clinic were included in the study.
Exclusion criteria
Children whose parents or caregivers did not give consent to participate in the study were excluded. Furthermore, excluded from the study were children below the age of 5 years because auto splenectomy is not expected, and they may not cooperate during the ultrasound scan (USS) procedure. Children with a history of blood transfusion within the previous 3 months and those on hydroxyurea therapy for whatever indication were also excluded from the study.
Ethical considerations
Approval was sought after and obtained from the Hospital Research and Ethics Committee of UMTH. Written informed consent was obtained from the caregivers after adequate education. Verbal assent was also obtained from children 7 years and older with unlimited liberty to deny consent/assent or opt out of the study at any time or stage without any consequences. Participation was, therefore, voluntary and those who refused consent to participate were still having their children or wards being followed up appropriately at the clinics. The information and results obtained were kept confidential.
Study procedure
The patients were identified and screened for eligibility by the study physician at the pediatric hematology clinic. Parents or guardians of patients who met the selection criteria were then approached for consent by the study physician and, in addition, assent was sought from children 7 years or older. Patients whose parents/guardians gave consent were then recruited and assigned a study number. A total of 180 children comprising 99 children with HSCD, within the age range of 5–15 years, and 81 apparently healthy children with HbAA matched for age, sex, and socioeconomic status with minor ailments on follow-up at the pediatric outpatient department were recruited as controls. Data from clinical history (from both parents/guardians and available in the clinical notes) were abstracted onto a pre-designed standard case record form. The descriptions included age at onset of symptoms, number of transfusions, hospitalizations, and severe pain episodes in the past year; complications such as cerebrovascular accidents, priapism, and avascular necrosis. Findings on physical examination were also documented.
Four milliliters of peripheral venous blood was drawn for a complete blood count and HbF levels. A drop of blood from the syringe was used for the preparation of a thick blood film for malaria parasites and the determination of parasite density.[17] The blood was then put in two ethylenediaminetetraacetic acids bottles; one for an automated complete blood count and the second for determination of HbF levels by the alkali denaturation method by Betke et al.[18] This test has been used in other studies of HbF levels in the Nigerian population by Fatunde and Scott-Emuakpor,[19] in the Senegalese population by Diop et al.,[20], and in Ugandan patients by Mpalampa et al.[21] This test utilizes the characteristic of HbF to resist denaturation in an alkaline solution than HbA. Alkali converts HbA to alkaline hematin. Alkaline hematin is insoluble and precipitates. HbF is quantitated by measuring the Hb concentration before and after denaturation. The procedure involves treating a cyanmethemoglobin solution with an alkaline reagent (sodium hydroxide). During this time, normal Hb is denatured or destroyed, but the HbF remains intact. Saturated ammonium sulfate is added to halt the denaturation process and to precipitate the denatured Hb. The solution is filtered, read at 420 nm, and measured spectrophotometrically and compared with the spectrochemistry readings of the original cyanmethemoglobin solution to determine the percentage of HbF present. All samples collected were analyzed on the collection day.
Malaria parasite detection
A thick blood film was prepared on a glass slide, and a smear preparation was made for malaria parasite quantification. This was air-dried and stained with 10% Giemsa solution and allowed to dry again. All blood smears were then examined microscopically using Olympus model CX21FS1 under oil immersion (×1,000 magnification), and the parasites were counted. Malaria parasite density quantification on the thick film was obtained by determining the number of parasites corresponding to a leukocyte count of 8000/microliter (µL) of blood.[22]
Parasite density was classified into mild (1–999/µL), moderate (1000–9999/µL), and severe (≥10,000/µL), while malaria hyperparasitemia was taken as parasite count of >250,000/µL.[23]
All the subjects had abdominal USS measurements for the longitudinal and coronal dimensions of the spleen. USS examination was done devoid of sedating the patients using a high-resolution real-time ultrasound scanner (Aloka SSD 3500, Japan) with a 3.5 MHz sector transducer. Each subject was scanned in a slightly right lateral decubitus position, precisely exposing the area of interest. Ultrasound gel was then applied to the area for optimization of the image. The spleen size was measured during deep inspiration to minimize the masking effect of the left lung. The longitudinal dimension of the spleen was measured in the oblique plane between the most superior medial and the most inferior lateral borders. The coronal dimension was obtained by measurement along with the 11th intercostal space. Values obtained were correlated with the body height which is considered the best criteria that correlate with the longitudinal dimension of the spleen.[24] The obtained spleen size was classified based on Konus et al., the reference value for spleen size in children.[24] Values between the 5th and 95th percentile are regarded as normal. A size greater than the 95th percentile was considered enlarged, while a size less than the 5th percentile was regarded as a shrunken spleen, and non-visualization in the absence of surgical splenectomy was regarded as auto splenectomy. The subjects studied were 180 Nigerian children, of which 99 were HSCD, and 81 were normal children with the HbAA Hb genotype, with all children within the age range of 5–15 years. All USSs were performed by the same radiologist to exclude observer variation. None of the subjects studied have had a surgical splenectomy in the past.
Data analysis
Data obtained were analyzed using the Statistical Package for the Social Sciences (SPSS) version 18 (SPSS, Chicago, Illinois, USA). Means, standard deviation, frequencies, and percentages were presented in tables and charts as appropriate. The significance of the difference between means values was determined using Student’s t-test. P < 0.05 at a 95% confidence interval was implied to have statistical significance.
RESULTS
A total of 180 children were recruited into the study; 99 (55%) were HbSS, while the remaining 81 (45%) were HbAA. The majority of children with HbSS 45 (57.7%) were in the age group 11–15 years compared to HbAA 27 (47.4%), who were mostly in the age group 2–5 years. There is no statistically significant difference in the age distribution between the two groups (χ2 = 0.408, P = 0.548). Furthermore, there was no significant difference in the mean age of the HSCD subjects (7.8 ± 3.96 years) and controls (7.92 ± 3.93 years), P = 0.852.
Fifty-one (54.8.4%) of the HbSS subjects and 42(45.2%) of the HbAA controls were male. There was no statistical difference in the gender distribution between the HbSS subjects and the HbAA controls (χ2 = 0.964, P = 0.542).
Fifty-four (85.7%) of the HbSS population were from the lower social class (Classes IV and V), whereas the middle (Class III) and upper (Classes I and II) classes constituted 66.7% and 30.0%, respectively. An opposite pattern of socioeconomic class distribution was observed among the controls, as shown in Table 1. This was statistically significant (χ2 = 48.225, P = 0.001).
| Variables | HbSS | HbAA | Total | χ2 | P |
|---|---|---|---|---|---|
| n(%) | n(%) | ||||
| Age | 0.408 | 0.548 | |||
| 2–5 | 30 (52.6) | 27 (47.4) | 57 | ||
| 6–10 | 24 (53.30) | 21 (46.7) | 45 | ||
| 11–15 | 45 (57.7) | 33 (42.3) | 78 | ||
| Sex | 0.964 | 0.542 | |||
| Male | 51 (54.8) | 42 (45.2) | 93 | ||
| Female | 48 (55.2) | 39 (44.8) | 87 | ||
| Social Class | 48.225 | 0.001* | |||
| Upper | 27 (30.0) | 63 (70.0) | 90 | ||
| Middle | 18 (66.7) | 9 (33.3) | 27 | ||
| Lower | 54 (85.7) | 9 (14.3) | 63 |
Table 2 shows the median (interquartile range) malaria parasite density between HbSS subjects and HbAA controls. Subjects with HbSS 440 (360–600) had a higher parasite density compared to HbAA controls 200 (200–360). This was statistically significant (U-stat= 1728, P = 0.001).
| Variables | Haemoglobin SS | Haemoglobin AA | U-Stat | P |
|---|---|---|---|---|
| Malaria Parasite Density Median (IQR) | 440 (360–600) | 200 (200–360) | 1728 | 0.001* |
Malaria parasite density progressively reduced with increasing age in HbSS subjects with normal spleen status, while the reverse is the case in those with abnormal spleen status. The difference was statistically significant, especially among the 6–10-year age group [Table 3].
| Variables | Splenic Status | U-stat | P | |
|---|---|---|---|---|
| Normal | Abnormal | |||
| Parasite Density Median (IQR) | ||||
| 2–5 years | 480 (400–600) | 360 (360–440) | 81.000 | 0.202 |
| 6–10 years | 380 (280–470) | 600 (510–960) | 4.500 | 0.001* |
| 11–15 years | 360 (360–420) | 480 (320–760) | 130.500 | 0.083 |
| Total | 400 (360–480) | 480 (360–680) | 814.500 | 0.010* |
Malaria parasite density appears to be lower in the age group 2–5 years with high HbF; however, the statistical test is not significant (H-test = 0.602, P = 0.740) [Table 4].
| Variable | HbF Median (IQR) | Malaria parasite density Median (IQR) | H-test | P |
|---|---|---|---|---|
| Age | 0.602 | 0.740 | ||
| 2–5 years | 6.8 (6.5–7.8) | 420 (360–600) | ||
| 6–10 years | 6.2 (5.8–7.1) | 480 (380–600) | ||
| 11–15 years | 6.2 (5.8–6.7) | 440 (360–680) | ||
| Total | 6.4 (5.8–7.0) | 440 (360–600) |
IQR: Interquartile range. HbF: Fetal hemoglobin
Figure 1 shows the negative and positive correlation between the 2–5-year age group and the 6–10-year age group with malaria parasite density, respectively, and no correlation with the 11–15-year age group for the HbSS subjects.
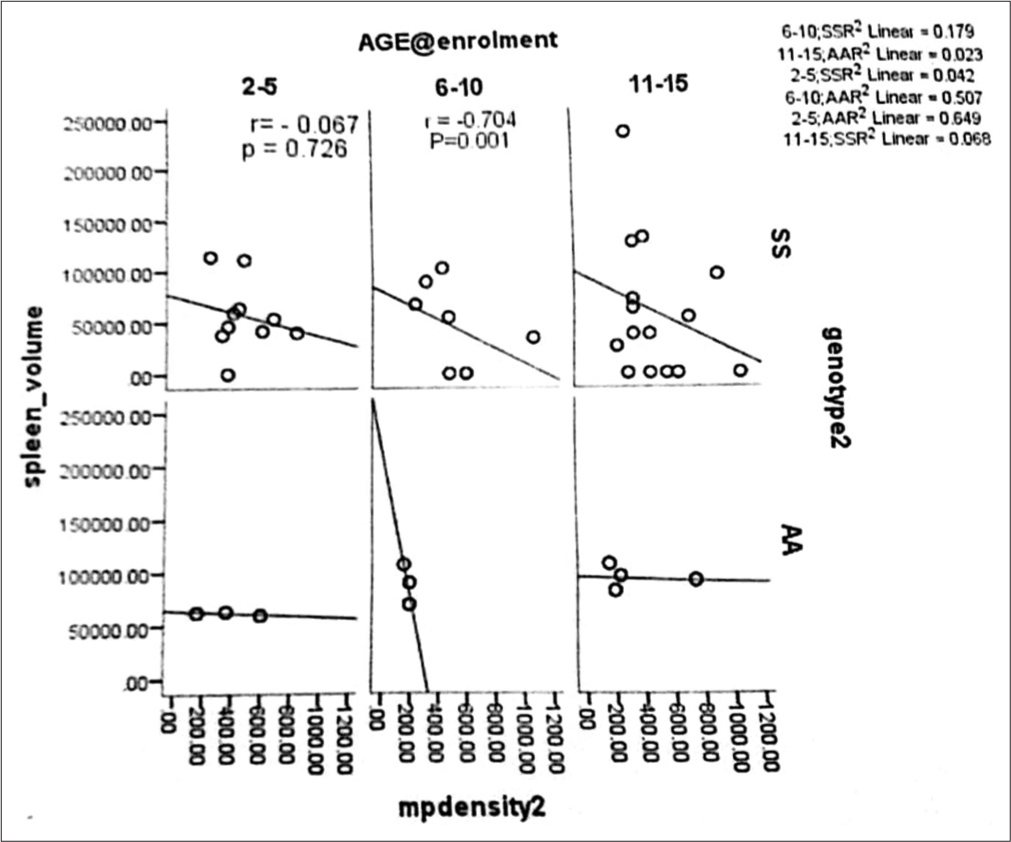
- Scatter plot correlating age group at enrolment, malaria parasite density, and spleen volume with Haemoglobin SS subjects and Haemoglobin AA.
Splenic size determination by palpation
The splenic size of the HSCD subjects by the palpatory method ranged from not palpable to 18 cm, whereas that of the normal HbAA subjects ranged from not palpable to 8 cm. The mean splenic size of the HbSS subjects was significantly higher than those of normal HbAA controls, t = 2.8089, P = 0.0055. Of the 99 HbSS subjects, 7 (7.1%) had splenomegaly. In 92 (92.9%) children with HbSS, the spleen was not palpable, 30 (32.6%) had splenic size of 1–5 cm determined by USS, 56 (60.9%) had size of 6–10 cm determined by USS, and 6 (6.5%) had spleen size >10 cm determined by USS. None of the HbAA controls had splenomegaly. There was a significant difference between the HbSS subjects with splenomegaly and the normal HbAA controls (Fisher’s exact; P = 0.0197).
DISCUSSION
Malaria has widely been a major cause of morbidity and mortality in children living in the malaria endemic zone of sub-Saharan Africa. It is also reported to be a major precipitant of both vaso-occlusive and hemolytic anemic crises.[25] This study was, however, conducted in a malaria-endemic region where there is usually a high rate of asymptomatic malaria parasitemia and inter-current infections with varieties of bacteria, viruses, and even other parasites, the presence of malaria parasitemia cannot be solely attributed to be responsible for splenomegaly.[26-30] Our research furnished us the opportunity to evaluate the possible contribution of malaria parasitemia to splenomegaly in asymptomatic SCA patients and compare our findings with healthy normal HbAA children with the same asymptomatic malaria parasitemia from the same environment. The malaria parasite density was significantly higher in the HbSS subjects compared to the HbAA group. The fact that malaria parasitemia was present in the SCA and HbAA groups who are both asymptomatic may imply that there is the presence of a reasonable degree of immunity to the malaria parasite among both populations. A higher malaria parasite density was noted among the younger (2–5 years) compared to the older SCA population with normal spleen. In contrast, among those with abnormal spleen status, the malaria parasite density showed an increase with increase in age (6–10 years) and a sharp drop in the older age group (11–15 years) but still lower than the younger patients aged 5 years and below. Similarly, the higher parasite density in those with abnormal splenic status showed statistical significance. Normally, age has been shown to be a vital factor that determines immunity among the population living in the malaria endemic zone; when an individual becomes infected with the parasite, the individual usually develops the symptomatology of acute malaria, which could be simple or complicated form depending on the burden of the infestation or the immunity of the individual. Recurrent episodes of infestation with the malaria parasite among help to develop clinical immunity to malaria through hyper-immune malaria splenomegaly. Therefore, individuals living in malaria endemic region are likely to have asymptomatic malaria infection.[25,31-33] Unfortunately, blood film for malaria parasite testing is not routinely done to determine asymptomatic individuals to treatment; hence, they continue to serve as reservoirs of the parasites and, therefore, contribute to the interminable spread of the parasite.[34] Our study was conducted in a malaria endemic region with a large variation in the clinical severity of malaria infection with the spectrum that ranges from asymptomatic parasitemia, simple febrile illness to complicated malaria (including hypoglycemia, severe anemia, pulmonary edema, acute kidney injury, hyperbilirubinemia, multiple convulsions, and cerebral malaria) and death at the end.[25,31] The observation of parasitemia among steady-state SCA patients is similar to the report from Nigeria among steady-state SCD controls.[25]
There has been increasing interest in issues relating to HbF level in children with HbSS over the past six decades as it has tremendous protective effects on the severity of SCD symptomatology and the timing of the beginning of the manifestation of the disease to the development of multiorgan dysfunction.[35] One of the objectives of the studies is to examine the relationship between malaria parasite density and HbF level among steady-state SCA children attending the sickle cell clinic in Maiduguri, Northeastern Nigeria. We found that the younger age group, 2–5 years, has relatively higher HbF levels and lower malaria parasite density. This finding is in concordance with the assertion that higher levels of HbF influence disease severity demonstrated by Mpalampa et al.[21] The overall average HbF in our patients was similar to what was observed in a Nigerian study by Fatunde and Scott-Emuakpor.[19] However, this finding is variant from the observation from other West African states, particularly Senegal that have a higher HbF levels.[20] This is however, not surprising because patients from Senegal are thought to have the Senegal haplotype, which is thought to be associated with a high HbF level.
HbF was identified to be a major SCD severity modulator factor.[26] In SCA, there is usually a delay in the genetically controlled postnatal switch of HbF to HbA, which consequently leads to a surge in HbF levels.[36] The precise mechanism for the delay in the switch of HbF to HbA is, however, unknown; it is speculated to be related to the accelerated expansion of early erythroid progenitor cells, which still possess the ability to express y-globin.[36] The accelerated progenitor cell expansion is directly linked to increased hemolysis and associated increased erythropoiesis in SCA.[26] Our finding of lower HbF in cohorts with SCA is lower than the reports of other researchers from Nigeria,[36,37] and similar developing nation.[38] The average HbF level of the cohort of children with HbSS in this research was higher than what had been reported from Sokoto northwest Nigeria where Isah et al.,[39] found an average HbF levels of 2.99% ± 5.16% as against 6.4% ± 0.6% in our study. This is despite the fact that the same Betke method of alkali denaturation was used. Furthermore, the finding of this study was higher than 2.17% ± 1.81% reported by Omoti in Benin, 4.26% ± 4.33% reported by Durosinmi in Ife, and 5.16% ± 4.04% reported by Olaniyi in Ibadan.[37,40,41] Age difference might account for the differences in the HbF levels obtained in our study, as those three were done in adults with SCA. In addition, a study among children with HbSS in Uganda by Mpalampa et al. [21] reported an average HbF level of 9.0% ± 5.58% despite using the same alkali denaturation test. A higher value of HbF (12.2% ± 7.1%) was equally reported among children with HbSS in India by Rao et al.[42] There was no reasonable explanation for the differences; it may, however, be due to the effect of various factors influencing the production of HbF in individuals with HbSS. One major identified factor is the beta gene globin haplotype, with the Indian, Saudi, and Senegal haplotypes generally associated with higher levels of HbF and milder disease course compared to the Benin and Cameroon haplotypes that are predominantly found in Nigeria with intermediate and of varying clinical course.[43] A powerful identified agent that affects the level of HbF is hydroxyurea, which is an antimetabolite hypomethylating cytotoxic agent that is currently being used all over the world in particular regions where SCA is prevalent in the management of patients with SCA. However, most patients in Northeastern Nigeria, where this study was conducted, are the most resource-constraint region with over a decade of Boko Haram insurgency and, incidentally, the region with the highest burden of SCA is not benefiting from the use of this Nobel drug that leads to surge of HbF and minimize the severe manifestation of SCA due to non-availability and unaffordability of the drug.[44] Other agent identified is a locally available fruit, Terminalia catappa (tropical almond); it was identified as an in vivo HbF-inducing agent.[45] This fruit was reported to be readily available and incidentally inexpensive, and a multicenter clinical trial of this agent was recommended by Akinlosotu et al.[36] Meanwhile, all hands must be put on the desk, and efforts must be put in place to ensure that hydroxyurea is made available and affordable to all SCA children and even adults in the Northeast insurgency-devastated region of Nigeria.
The spleen is a key reticuloendothelial intra-abdominal organ routinely evaluated by ultrasonography that plays a major role in the morbidities and mortalities seen in children with SCA. Persistent splenomegaly in Sub-Saharan African patients with SCA has been widely reported as a result of malaria endemicity.[36,40,46-48] In this study, we observed a progressive decrease in parasite density with increasing age in those with normal splenic status compared to children with shrunken and/or enlarged spleen and a significantly higher parasite density in children with abnormal splenic status. In the present study, the older age group subjects with splenomegaly had higher malaria parasite density. This finding is not consistent with reports by Akinlosotu et al.[36] from Nigeria and Sadarangani et al.[47] from Kenya, East Africa. It has also been suggested that this finding is more frequently seen in areas of malaria endemicity where persistent splenomegaly syndrome is more prevalent.[49] Furthermore, our finding is consistent with a report from Ile-Ife, Nigeria, where Adekile et al. reported a significant positive correlation between splenic size and malaria-specific immunoglobulin G and immunoglobulin M antibodies in children with HbSS.[35] Adekile et al. hypothesized that persistent splenomegaly in Nigerian children with HbSS is prevalent due to recurrent malaria infestation. However, studies in adult Nigerians with HbSS found no positive correlation between malaria parasitemia and splenic size.[40,46] Our study found no significant relationship between malaria parasite density and HbF levels in the study subjects with HbSS. The precise reason for persistent splenomegaly in SCA patients is still a subject of research that further studies are desirable in the light of the possibility of finding multiple factors that may be confounders to the established malaria endemicity in Sub-Saharan Africa. Conceivably, the extramedullary erythropoiesis, splenic sequestration, occasional congestion, and recurrent infectious diseases in Sub-Saharan Africa are likely contributory factors.
CONCLUSION
Children with HbSS had a higher malaria parasite density than matched HbAA controls, although the children were in a steady state. It also appears that a significant relationship exists between malaria parasite density and spleen size in children with HSCD. In addition, HbF level was not significantly related to asymptomatic malaria parasitemia in a steady state. Furthermore, our patients had lower mean HbF across all ages. It is, therefore, recommended that early introduction of hydroxyurea to the care of SCA children will be necessary in the routine care of children with SCA to reduce morbidity and mortality from confounding infectious diseases, including Malaria.
Ethical approval
The research/study is approved by the Hospital Research and Ethics Committee of University of Maiduguri Teaching Hospital (UMTH) dated: 15 October 2012.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Management of birth defects and haemoglobin disorders: Report of a joint WHO-March of Dimes meeting. 2006. Geneva: World Health Organization; Available from: https://iris.who.int/handle/10665/43587 [Last accessed on 2021 Apr 04]
- [Google Scholar]
- Abnormal haemoglobins in the Sudan savanna of Nigeria. I. Prevalence of hemoglobins and relationships between sickle cell trait, Malaria, and survival. Ann Trop Med Parasitol. 1979;73:161-72.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of hemoglobinopathy and malaria disease in the population of old Aguata division, Anambra state, Nigeria. Biokemistri. 2013;15:57-66.
- [Google Scholar]
- A case of sickle-cell anaemia: A commentary on 'abnormal human haemoglobins. I. The comparison of normal human and sickle-cell haemoglobins by 'fingerprinting" with II. The chymotryptic digestion of the trypsin-resistant 'core' of haemoglobins A and S and III. The chemical difference between normal and sickle cell haemoglobins. Biochim Biophys Acta. 1989;1000:147-50.
- [Google Scholar]
- Clinical features of genetic variants of sickle cell disease. Bull John Hopkins Hosp. 1954;94:289-318.
- [Google Scholar]
- Studies in sickle cell anaemia XXVII: Complications in infants and children in the United States. Clin Pediatr. 1966;5:403-99.
- [CrossRef] [PubMed] [Google Scholar]
- Natural history of sickle cell disease-the first ten years. Semin Hematol. 1975;12:267-85.
- [Google Scholar]
- Persistent gross splenomegaly in Nigerian patients with sickle cell anaemia: Relationship to Malaria. Ann Trop Paediatr. 1988;8:103-7.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical spectrum of sickle cell anaemia in Nigerian adults. INSERM. 1975;44:43-50.
- [Google Scholar]
- Clinical aspects of sickle cell disease in Nairobi children. Am J Pediatr Hematol Oncol. 1982;4:187-90.
- [Google Scholar]
- Sickle cell anaemia in children in Eastern Nigeria:. A detailed analysis of 210 cases. East Afr Med J. 1982;59:742-9.
- [Google Scholar]
- Encyclopedia of animal cognition and behaviours United States: Springer International Publishing, AG; 2017.
- [Google Scholar]
- The protection afforded by sickle-cell trait against Subtertian malarial infection. Br Med J. 1954;1:290-4.
- [CrossRef] [PubMed] [Google Scholar]
- Heterozygote advantage as a natural consequence of adaptation in diploids. Proc Natl Acad Sci USA. 2011;108:20666-71.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of small percentages of foetal fobin. Nature. 1959;184(Suppl 24):1877-8.
- [CrossRef] [PubMed] [Google Scholar]
- Foetal haemoglobin in Nigerian children with sickle cell anaemia. Effect on haematological parameters and clinical severity. Trop Geogr Med. 1992;44:264-6.
- [Google Scholar]
- New results in clinical severity of homozygous sickle cell anaemia in Dakar, Senegal. Hematol Cell Ther. 1999;41:2217-21.
- [CrossRef] [PubMed] [Google Scholar]
- Foetal haemoglobin and disease severity in sickle cell anaemia patients in Kampala, Uganda. BMC Hematol. 2012;12:11.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of two simple methods for determining malaria parasite density. Trans R Soc Trop Med Hyg. 1991;85:186-8.
- [CrossRef] [PubMed] [Google Scholar]
- Guideline for the treatment of malaria (2nd ed). Geneva: World Health Organization; 2010.
- [Google Scholar]
- Normal liver, spleen and kidney dimensions in neonates, infants and children: Evaluation with sonography. AJR Am J Roentgenol. 1998;171:1693-8.
- [CrossRef] [PubMed] [Google Scholar]
- Malaria infection in patients with sickle cell disease in Nigeria: Association with markers of hyposplenism. Hemoglobin. 2024;48:15-23.
- [CrossRef] [PubMed] [Google Scholar]
- Acute illness in Nigerian children with sickle cell anaemia. Ann Trop Paediatr. 1987;7:181-6.
- [CrossRef] [PubMed] [Google Scholar]
- Acute sickle cell syndrome in Nigerian adults. Clin Lab Haematol. 2000;22:151-5.
- [CrossRef] [PubMed] [Google Scholar]
- Associated morbidities in children with sickle-cell anaemia presenting with severe anaemia in a malarious area. Trop Doct. 2001;31:26-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentation of severe anaemia in pediatric patients with sickle cell anemia seen in Enugu, Nigeria. Am J Hematol. 2003;72:185-91.
- [CrossRef] [PubMed] [Google Scholar]
- A global network for investigating the genomic epidemiology of Malaria. Nature. 2008;456:732-7.
- [CrossRef] [PubMed] [Google Scholar]
- Severe childhood malaria in two areas of markedly different falciparum transmission in east Africa. Acta Trop. 1994;57:289-300.
- [CrossRef] [PubMed] [Google Scholar]
- Naturally acquired immunity to Plasmodium falciparum. Parasitol Today. 1991;7:68-71.
- [CrossRef] [Google Scholar]
- Premunition in Plasmodium falciparum infection: Insight from the epidemiology of multiple infections. Trans R Soc Trop Med Hyg. 1999;93(Supplement 1):59-64.
- [CrossRef] [PubMed] [Google Scholar]
- Spleen in sickle cell anaemia: Comparative study of Nigerian and U.S. patients. Am J Haematol. 1993;42:316-21.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal hemoglobin level and its relationship with spleen size and malaria parasite density in Nigerian children with sickle cell anemia. Ann Trop Med Public Health. 2018;11:133-9.
- [CrossRef] [Google Scholar]
- The value of foetal haemoglobin level in the management of Nigerian sickle cell anaemia patients. Niger Postgrad Med J. 2005;12:149-54.
- [CrossRef] [PubMed] [Google Scholar]
- Foetal haemoglobin, erythrocytes containing foetal haemoglobin, and hematological features in congolese patients with sickle cell anaemia. Anemia. 2012;2012:105349.
- [CrossRef] [PubMed] [Google Scholar]
- Foetal haemoglobin levels in sickle cell disease patients in Sokoto, Nigeria. Br J Med Health Sci. 2013;1:36-47.
- [Google Scholar]
- Haematological parameters in sickle cell anaemia patients with and without splenomegaly. Niger Postgrad Med J. 2005;12:271-4.
- [CrossRef] [PubMed] [Google Scholar]
- Foetal haemoglobin (HbF) status in adult sickle cell anaemia patients in Ibadan, Nigeria. Ann Ib Postgrad Med. 2010;8:30-3.
- [CrossRef] [PubMed] [Google Scholar]
- Hematological profile of sickle cell disease from South Gujarat. India Hematol Rep. 2012;4:e8.
- [CrossRef] [PubMed] [Google Scholar]
- Hematologically and genetically distinct forms of sickle cell anemia in Africa. The Senegal type and the Benin type. N Engl J Med. 1985;312:880-4.
- [CrossRef] [PubMed] [Google Scholar]
- Current sickle cell disease management practices in Nigeria. Int Health. 2014;6:23-8.
- [CrossRef] [PubMed] [Google Scholar]
- Introduction of foetal haemoglobin synthesis in erythroid progenitor stem cells: Mediated by water-soluble components of Terinalia catappa. Cell Biochem Funct. 2014;32:361-7.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency of hepatomegaly and splenomegaly in Nigerian patients with sickle cell disease. West Afr J Med. 2007;26:274-7.
- [CrossRef] [PubMed] [Google Scholar]
- An observational study of children with sickle cell disease in Kilifi, Kenya. Br J Haematol. 2009;146:675-82.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of sickle cell anemia in a pediatric environment in Gabon. Sante Publique. 1997;9:45-60.
- [Google Scholar]
- Autosplenectomy of sickle cell disease in Zaria, Nigeria: An ultrasonographic assessment. Oman Med J. 2012;27:121-3.
- [CrossRef] [PubMed] [Google Scholar]







