Translate this page into:
Oxygen dissociation curve characteristics of common hemoglobin variants and its potential implications: A brief report
*Corresponding author: Rakhee Kar, Department of Pathology, Jawaharlal Institute of PostGraduate Medical Education and Research, Puducherry, India. drrakheekar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kuppusamy D, Nanda N, Delhi Kumar CG, Vinod KV, Mandal AK, Kar R. Oxygen dissociation curve characteristics of common hemoglobin variants and its potential implications: A brief report. J Hematol Allied Sci. 2025;5:61-5. doi: 10.25259/JHAS_41_2024
Abstract
Objectives:
Hemoglobin E and S (HbE and HbS) are the most common hemoglobin (Hb) variants widely spread across the Asian continent that differ from the normal adult Hb in a single amino acid in the β-globin gene. The aim of this study was to explore if this change in globin structure has any effect in the function of Hb in terms of its oxygen affinity.
Material and Methods:
The oxygen dissociation curve (ODC) describes the relationship between the partial pressure of oxygen (pO2) and oxygen saturation of hemoglobin (sO2). ODC was plotted based on pO2 and sO2 values obtained on bubbling buffered oxygen and nitrogen gases through reconstituted variant Hb mixture samples in an experimental setup.
Results:
The p50 values of fetal hemoglobin (HbF), HbA, HbE, Sbeta, and HbS were found to be 20 ± 2 mmHg, 28.01 ± 2.5 mmHg, 33 ± 1.5 mmHg, 38 ± 2 mmHg, and 40± 0.6 mmHg at a pH of 7–7.5 and ambient temperature of 25°C, respectively.
Conclusion:
HbF had greater oxygen affinity while HbE and HbS showed a decreased oxygen affinity, more pronounced with HbS, possibly due to shift in the equilibrium toward the deoxy form. Even though the clinical implication may be variable, these findings reflect that there could be an interplay between Hb variants and oxygen affinity.
Keywords
Hemoglobin
Hemoglobin E
Hemoglobin S
Oxygen dissociation curve
p50
Partial pressure of oxygen
Oxygen saturation of hemoglobin
INTRODUCTION
Hemoglobin (Hb) is an iron-containing respiratory pigment in the red blood cells whose major function is to transport oxygen in the form of oxyhemoglobin from the lungs and deliver it to the tissues and to a lesser extent facilitate the return of carbon dioxide in the form of carbaminohemoglobin acting as a two-way carrier. Adult Hb comprises two alpha (α) and two beta (β) homologous polypeptide chains consisting of 141 and 146 amino acid residues, respectively, with each chain containing heme as a prosthetic group. Heme has one ferrous atom at the center of a protoporphyrin ring that reversibly binds one oxygen molecule.[1] Hb as a heterotetramer exhibits cooperative binding wherein the binding of one oxygen molecule to heme in one of the subunits increases the subsequent binding affinity of oxygen molecules in the other subunits.[2] Hb in the absence of oxygen is referred to as T or Taut (Tense) form where constraints between subunits oppose the structural changes resulting from ligand binding. When the oxygen is bound to the Hb, it is in a Relaxed (R) state where these constraints are released, thus enhancing ligand-binding affinity. Allosteric effects in Hb arise from an equilibrium between these energy states of the molecule.[3]
The oxygen dissociation curve (ODC) describes the relationship between the partial pressure of oxygen (pO2) and oxygen saturation of hemoglobin (sO2). The cooperative binding of oxygen to Hb results in the sigmoid-shaped ODC.[4,5] It depends on several factors such as pH, temperature 2,3 bisphosphoglycerate, and carbon dioxide.[1,6] p50 is the pO2 in blood in which hemoglobin is 50% saturated; any change in this value therefore reflects whether the curve has shifted to the left or the right.[1] A rightward shift indicates that the Hb under study has a decreased affinity for oxygen whereas left shift of the curve is a sign of Hb’s increased affinity for oxygen.[1,5] The study of the ODC helps us to understand the various factors affecting oxygenation in patients. A decrease in oxygen affinity due to disease mechanisms and therapeutic approaches can also be identified. This may also help to identify the effect of factors affecting the oxygen affinity of Hb in critically ill patients.[7]
The most prevalent Hb fraction in adults is hemoglobin A (HbA). In conditions such as thalassemia and hemoglobinopathies, there is a change in the fraction of Hbs with either increased amounts of fetal hemoglobin (HbF) in homozygous β-thalassemia or the appearance of variant Hb fractions in various hemoglobinopathies. The common variant Hbs in our population is HbS and HbE.[8] Given the established distinctions of HbS and HbE, a comparison of these Hb variants’ affinities for oxygen as indicated by the oxyhemoglobin dissociation curve would be beneficiary to indicate if they have a potential effect on tissue oxygenation. Hence, this study was undertaken to study the ODC characteristics of normal and common variant Hbs in our patient population.
MATERIAL AND METHODS
Study setting
This was a descriptive cross-sectional study conducted in an experimental setup in the hematology section of Pathology Department in a tertiary-care hospital in southern India. It commenced after the approval of the study by the Institute Scientific Advisory (JSAC/59/2018/175) and ethics (JIP/IEC/2018/0283) committees.
Sample collection and processing
Approximately 2 mL of whole blood of routine ethylenediaminetetraacetic acid (EDTA) samples with the presence of known HbF (newborn samples), HbA (adult sample), HbE (HbE trait patients), and HbS (sickle cell hemoglobinopathy patients) were collected. SBeta was also an HbS predominant sample; it was pooled from HbS β-thalassemia samples unlike HbS which was from sickle cell anemia samples. These samples had been sent to the hematology section for complete blood count/hemogram and Hb-high-performance liquid chromatography (HPLC) studies in EDTA vacutainers. All these samples were subjected to Hb-HPLC (Biorad D10) to identify the concentrations of various Hb fractions (Hb A, F, E, and S, respectively) and stored at 4°C. Two to three samples of similar Hb fractions were pooled to obtain a sample volume of 4–6 mL for the experiment and the final composition of Hb fractions was checked by Hb HPLC [Table 1].
| S. No. | Reconstituted sample ID | Primary Hb fraction (%) | Other major Hb if any (%) | Final Hb constitution |
|---|---|---|---|---|
| 1. | HbF | HbF (60) | HbA (35) | HbFA |
| 2. | HbA | HbA (90) | – | HbA |
| 3. | HbE | HbE (27) | HbA (55) | HbAE |
| 4. | SBeta | HbS (63) | HbF (22), HbA2(3.8) | HbSF |
| 5. | HbS | HbS (72) | HbF (15) | HbSF |
HbF: Fetal hemoglobin, HbA: Hemoglobin A, HbE: Hemoglobin E, HbS: Hemoglobin S, SBeta: Sickle beta thalassemia, HbA2: Hemoglobin A2, HbSF: Hemoglobin S and F
Experimental setup
An experimental setup was constructed using plastic syringes, a three-way plastic cannula, and plastic tubing. An aliquot of the Hb mixture was taken in the syringe. After adding sodium dithionite, the samples were subjected to bubbling in different concentrations of buffered (with 5.4% carbon dioxide) oxygen and nitrogen gases gradually.[9,10] Without exposure to further atmospheric oxygen, a drop of a sample of the whole blood after bubbling was used immediately to find the pO2 and oxygen saturation of hemoglobin (sO2) using a point-of-care device (Epoc blood analysis system, Siemens). The values noted were the pO2, sO2, pH, partial pressure of carbon dioxide (pCO2), and sodium ion, respectively. Using the pressure valve regulators, the proportion of buffered oxygen and nitrogen from the two gas cylinders was altered to have a gradient of oxygen starting from low to high. Different aliquots were used for each round of bubbling. Based on the values of pO2 and sO2 obtained for each reconstituted Hb, ODC was plotted and the p50 value was calculated. The data have been presented as observed values for single readings and summary data for multiple readings.
RESULTS
On plotting pO2 against sO2 of HbF, HbA, HbE, SBeta, and HbS variants, the results obtained were a sigmoid curve. The observed p50 of HbF, HbA, HbE, Sbeta, and HbS was found to be 20 ± 2 mmHg, 28.01 ± 2.5 mmHg, 33 ± 1.5 mmHg, 38 ± 2 mmHg, and 40 ± 0.6 mmHg at a pH of 7–7.5 and ambient temperature of 25°C, respectively. The mean values of the electrolytes and other parameters for the varying Hb concentration mixtures are presented in Table 2. The ODC of all variant Hbs was in sigmoid shape on plotting pO2 against sO2 with HbF showing a left shift and variant Hbs showing a rightward shift to HbA. The superimposed ODC showing the different Hb fractions is shown in Figure 1.
| Sample ID | p50 | pH | Na+ (mmoL/L) | K+ (mmoL/L) | Cl− (mmoL/L) | Hct (%) | Glucose (mg/dL) | Lactate (mmoL/L) | Creatinine (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|
| HbF | 20 | 6.72 | 112.8 | 12 | 136.2 | 28 | 20.2 | 6.34 | 0.3 |
| HbA | 28 | 7.2 | 117.3 | 12 | 116.9 | 18.75 | 47.7 | 6.3 | 0.3 |
| HbE | 33 | 7.09 | 134.9 | 11.41 | 123.6 | 31.2 | 66.5 | 2.92 | 0.35 |
| SBeta | 38 | 7.015 | 121.7 | 12 | 123.72 | 26.57 | 20 | 6.15 | 0.39 |
| HbS | 40 | 7.05 | 121.8 | 12 | 118.2 | 15.8 | 86.4 | 4.1 | 0.4 |
HbF: Fetal hemoglobin, HbA: Hemoglobin A, HbE: Hemoglobin E, HbS: Hemoglobin S, Na+: Sodium ion, K+: Potassium, Cl−: Chlorine ion, Hct: Hematocrit
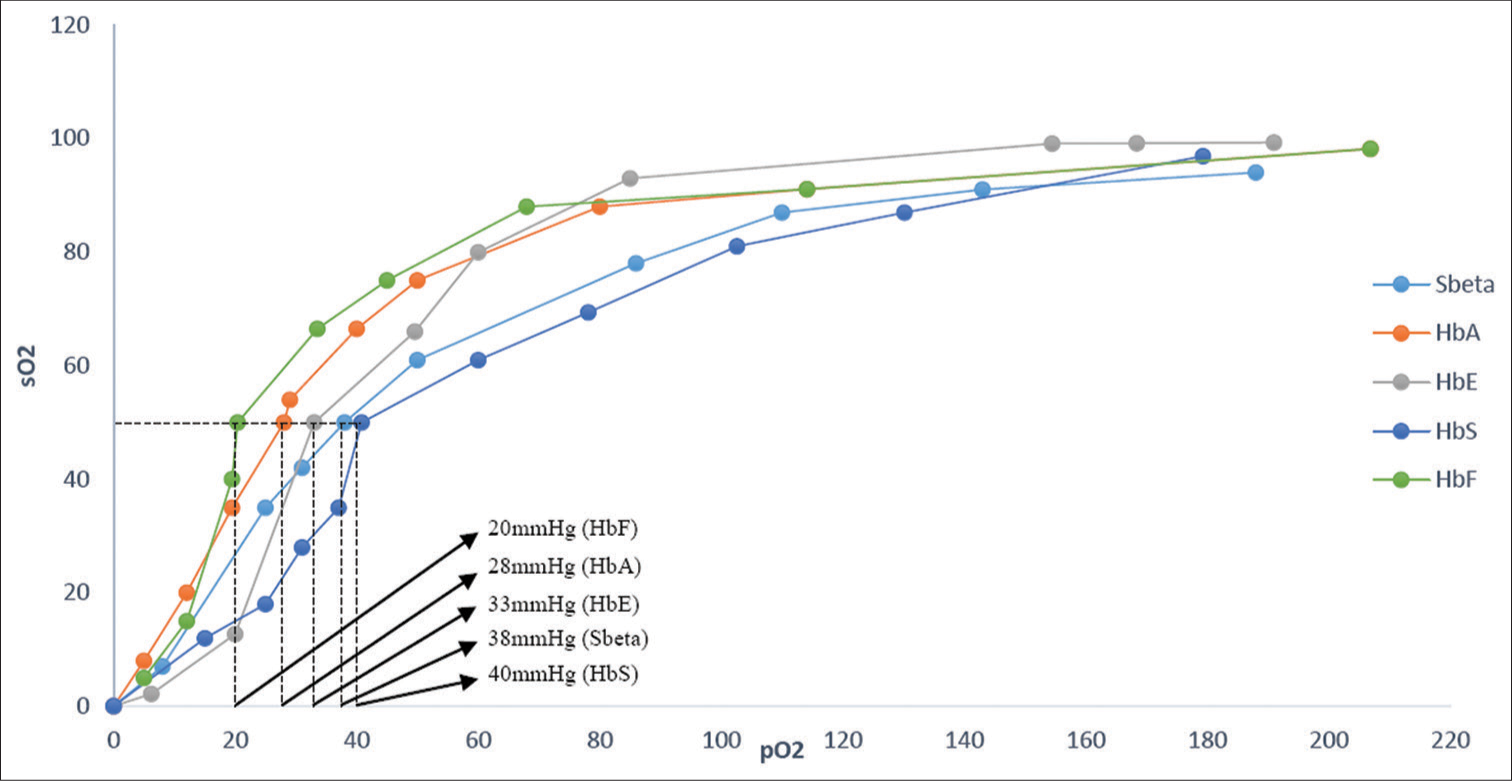
- Oxygen dissociation curve characteristics of the various hemoglobins showing higher oxygen affinity of fetal hemoglobin predominant sample and lower oxygen affinity of hemoglobin S and hemoglobin E containing samples relative to hemoglobin A. HbF: Fetal hemoglobin, HbA: Hemoglobin A, HbE: Hemoglobin E, HbS: Hemoglobin S, SBeta: Sickle beta thalassemia, sO2: oxygen saturation of hemoglobin, pO2: partial pressure of oxygen.
DISCUSSION
In this study, we examined the structure-function relationship of common human Hbs (HbA, HbF) and common Hb variants (HbE and HbS) to explore the effect of point mutations on the functionally active conformation of variant Hb molecules. In the ODC experiment using pooled samples containing HbF, HbA, HbE, and HbS as primary fractions, the variants, especially HbS, showed higher p50 values, indicating a decrease in the oxygen affinity relative to HbA. The observed p50 values for HbF, HbA, HbE, SBeta, and HbS were found to be 20 mmHg, 28 mmHg, 33 mmHg, 38 mmHg, and 40 mmHg. The p50 values obtained for HbA and the variant Hb mixtures corroborated with those reported in previous studies.[6,10,11]
In a previous study on the structure function relationship of variant Hbs, HbE, and HbD, there was a decrease in the oxygen affinity of the variants in comparison to HbA evidenced by an increase in p50 values. It was observed that the deoxy forms of these Hbs were more stabilized. The decrease in oxygen affinity suggested that the oxygen dissociation equilibrium was more pushed toward Hb’s deoxy form.[10]
The right shifted ODC of SCD has been thought to result in abnormally low sO2, even when pO2 is normal. SCD patients do have elevated p50, indicating reduced Hb oxygen affinity.[12] The sickle polymer has much lower affinity for oxygen than HbA or HbS when it is in solution.[13,14] Since HbS polymerization is directly linked to the severity of hypoxemia, oxygen affinity would be expected to be normal when arterial oxygenation is normal. Hence, it is believed that even among patients with right-shifted p50, the oxygen affinity of Hb remains normal at normal arterial pO2.[12] The higher p50 facilitates oxygen unloading and provides an explanation to why the patients can tolerate chronic severe anemia quite well. On the other hand, a higher p50 also favors the formation of deoxyhemoglobin, which consequently increases the polymerization of HbS and may trigger a sickling crisis if the peripheral pH drops.[6]
In comparison to HbA, HbF showed a leftward shift of the curve indicating a higher oxygen affinity which is already established. HbF is structurally different than adult Hb having two alpha and two gamma chains. The gamma chains have a reduced affinity for 2,3-DPG. Hence, HbF has a higher affinity for oxygen at lower levels of partial pressure and resulting in a leftward shift of the dissociation curve. HbF is the principal oxygen carrier in preterm and term neonates. The left shifted curve is advantageous as the fetus can pull oxygen from maternal circulation at placental interphase with greater ease. There is also a lower oxygen extraction at capillary beds in peripheral tissues.[15,16]
The equilibrium between the functional, active conformation of Hb’s deoxy and oxy forms determines Hb’s oxygen affinity. Modifiers and other factors could also affect the functional capacity. The study has certain limitations: It was conducted in an experimental setup and the Hbs studied were not in pure form but constituted a predominant or sizable fraction in the mixture. However, the varying proportion of HbA and HbF in these mixtures could be a confounding factor. Unfortunately, we were unable to measure 2,3 PBPG and temperature with the instrument used in the experiment and mild variations were observed in the pCO2 values.
CONCLUSION
The study of ODC characteristics offers a way to understand the structure-function relationship of Hb. The varying oxygen binding affinities of HbE and HbS in contrast to HbA in this study reflect the interplay between Hb variants and oxygen affinity. Thus, ODC analysis can serve as an adjunctive methodology to understand functional consequence of a structural variation in Hb, providing further perspectives when employed in conjunction with established diagnostic techniques.
Ethical approval
The research/study was approved by the Institutional Review Board at Jawaharlal Institute of Postgraduate Medical Education and Research, number JIP/IEC/2018/0283, dated 10th August 2018.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: JIP/Res/Intramural/Phs2/2018-2019 dated 19-1-2019.
References
- Physiology of haemoglobin. Contin Educ Anaesth Crit Care Pain. 2012;12:251-6.
- [CrossRef] [Google Scholar]
- An origin of cooperative oxygen binding of human adult hemoglobin: Different roles of the a and b subunits in the a2b2 tetramer. PLoS One. 2015;10:e0135080.
- [CrossRef] [PubMed] [Google Scholar]
- A signature of the T---> R transition in human hemoglobin. Proc Natl Acad Sci U S A. 2001;98:3773-7.
- [CrossRef] [PubMed] [Google Scholar]
- Relating oxygen partial pressure, saturation and content: The haemoglobin-oxygen dissociation curve. Breathe. 2015;3:194-201.
- [CrossRef] [PubMed] [Google Scholar]
- Physiology, oxyhemoglobin dissociation curve In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499818 [Last accessed on 2024 Jul 28]
- [Google Scholar]
- Respiratory function of hemoglobin. N Engl J Med. 1998;33:239-47.
- [CrossRef] [PubMed] [Google Scholar]
- The oxyhaemoglobin dissociation curve in critical illness. Crit Care Resusc. 1999;1:93-100.
- [CrossRef] [PubMed] [Google Scholar]
- Haemoglobinopathies in tribal populations of India. Indian J Med Res. 2015;141:505-8.
- [Google Scholar]
- Conjugation of para-benzoquinone of cigarette smoke with human hemoglobin leads to unstable tetramer and reduced cooperative oxygen binding. J Am Soc Mass Spectrom. 2018;29:2048-58.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of point mutation on structure-function correlation of hemoglobin variants, HbE and HbD Punjab. Amino Acids. 2020;52:893-904.
- [CrossRef] [PubMed] [Google Scholar]
- A broad diversity in oxygen affinity to haemoglobin. Sci Rep. 2020;10:16920.
- [CrossRef] [PubMed] [Google Scholar]
- The oxygen affinity of sickle hemoglobin. Respir Physiol Neurobiol. 2008;161:92-4.
- [CrossRef] [PubMed] [Google Scholar]
- Direct intracellular measurement of deoxygenated hemoglobin S solubility. Blood. 2001;98:883-4.
- [CrossRef] [PubMed] [Google Scholar]
- Oxygen binding to sickle cell hemoglobin. J Mol Biol. 1979;130:175-89.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal hemoglobin and tissue oxygenation measured with near-infrared spectroscopy-a systematic qualitative review. Front Pediatr. 2021;9:710465.
- [CrossRef] [PubMed] [Google Scholar]
- Physiology, fetal hemoglobin In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
- [Google Scholar]







