Translate this page into:
Rare cytogenetic subtype in a case of Ewing sarcoma with diagnostic dilemma: A case report
*Corresponding author: Ravikiran Narayansing Pawar, Department of Oncopathology, Homi Bhabha Cancer Hospital and Research Centre, Punjab, India. ravikiransingh2512@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pawar RN, Ray D, Sancheti S, Somal PK, Nishith N, Prasad P, et al. Rare cytogenetic subtype in a case of Ewing sarcoma with diagnostic dilemma: A case report. J Hematol Allied Sci. 2025;5:96-8. doi: 10.25259/JHAS_52_2024
Abstract
Ewing sarcoma is aggressive tumor with round cell morphology and very common in <15 years of age and young adults. Overall, incidence ranges from 10 to 15% of all the bone sarcomas. Ewing sarcoma most commonly affects lower extremity especially femur and other sites includes pelvis, upper extremity, and axial skeleton and ribs. The tumor cells are round blue cells on routine microscopy and the close differential includes other soft-tissue tumors such as primitive neuroectodermal tumor and neuroepithelioma. In addition to histology, the ancillary tests such as immunohistochemistry and cytogenetics studies are important for confirm diagnosis. Ewing sarcoma characteristically shows involvement of EWS gene located on chromosome 22q12. The most frequent translocation is EWS::FLI1 [t(11;22)(q24;q12) which appears approximately 85% of the cases followed by rare subtypes. Here, we report a case of young male who presented with fever, low backache, and weakness in lower limbs since 20 days. On CBC profile was normal and no significant past history; radiologically, there was mild-to-moderate compression fracture of L4 vertebral body with associated mild-to-moderate retropulsion of the posterior portion directly indenting and compressing the descending intrathecal nerve roots on both sides with small to moderate sized adjacent soft-tissue component. Diffuse alteration of marrow signal intensity involving cervical, dorsal, lumbar, and sacral vertebral bodies s/o? leukemia/lymphoma. Bone marrow and trephine biopsy suggested non hematopoietic malignancy. Cytogenetics studies showed aneuploidy (48,XY,+4,+8,t[9;22;21]); on that basis considered myeloid neoplasm with BCR:: ABL1 fusion. Which further on fluorescent in situ hybridization (FISH) considered negative. The final diagnosis was favored Ewing’ sarcoma based on the immunohistochemistry and cytogenetic translocation of chromosome 21 and 22. We report this case to highlight the importance of FISH as well as conventional karyotype for the additional chromosomal abnormalities in the Ewing’ sarcoma which, in isolation, leads to diagnostic dilemma.
Keywords
Aneuploidy
Cytogenetic
EWS fusion
INTRODUCTION
Ewing sarcoma is aggressive tumor with round cell morphology and very common in <15 years of age and young adults. Overall, incidence ranges from 10 to 15% of all the bone sarcomas.[1] Ewing sarcoma most commonly affects lower extremity especially femur and other sites include pelvis, upper extremity, and axial skeleton and ribs.[2] The tumor cells are round blue cells on routine microscopy and the close differential includes other soft-tissue tumors such as primitive neuroectodermal tumor and neuroepithelioma. In addition to histology, the ancillary tests such as immunohistochemistry and cytogenetics studies are important for confirm diagnosis.
Ewing sarcoma characteristically shows involvement of EWS gene located on chromosome 22q12. The most frequent translocation is EWS::FLI1 [t(11;22)(q24;q12)] which appears approximately 85% of the cases followed by rare subtypes.[3] The other subtypes found in 15% of tumors, with the t(21;22)(q22;q12) translocation resulting in fusion of EWS with the ERG gene on 21q22 being the second most common. Various other translocations are described with chromosome 22 such as other variant translocations have been described with translocation of chromosome 22 with chromosome 7, 17, and 2. Many complex translocation are documented with three chromosomes involved such as translocation of chromosome 11, 14, and 22 (t[11;14;22]) and with chromosome 10, 11, and 22.[4-6] The importance of genetic or translocation subtypes is not yet documented with clinical importance. However, these are the useful in diagnosis and proper management of these cases. On treatment part, these cases are managed by neoadjuvant chemotherapy followed by surgery and recent trials are for immunotherapy are being considered for the better outcomes.[7]
CASE REPORT
A 29-year-old male, symptomatic for one month intermittent fever, low back ache, and weakness in bilateral lower limbs since 20 days. On laboratory evaluation, hemogram was normal with mild anemia and leucoerythroblastic picture other biochemical work up was also in normal limits. On clinical examination, there was pallor with no evidence of icterus and edema, there was no palpable lymphadenopathy and no hepatosplenomegaly. Radiology screening with magnetic resonance imaging spine shows mild-to-moderate compression fracture of L4 vertebral body with associated mild-to-moderate retropulsion of the posterior portion directly indenting and compressing the descending intrathecal nerve roots on both sides with small to moderate sized adjacent soft-tissue component. Diffuse alteration of marrow signal intensity involving cervical, dorsal, lumbar and sacral vertebral bodies suggests the possibility of leukemia or lymphoma.
On this basis, marrow was planned and sent for flowcytometry and cytogenetics evaluation. Bone marrow aspiration smears show an excess of atypical cells with focal aggregates, indicating the possibility of metastatic deposits [Figure 1]. The additional sample of bone marrow biopsy also reveals diffuse involvement by round blue cells [Figure 2]. On immunohistochemistry, these cells are positive for CD99 and focally by NKX2.2 and negative for CD45, TdT, and PAX5 [Figure 3]. The possibility of Ewing’s sarcoma was considered with advise of genetic study. The cytogenetics report suggest the presence of complex translocation t(9,22,21) along with gain of chromosome 4 and 8 which mislabeled as a myeloproliferative neoplasm. However, in view of above findings, the FISH for BCR ABL1 was found to be negative and later considered as a rare rearrangement with EWS gene located on chromosome 22.
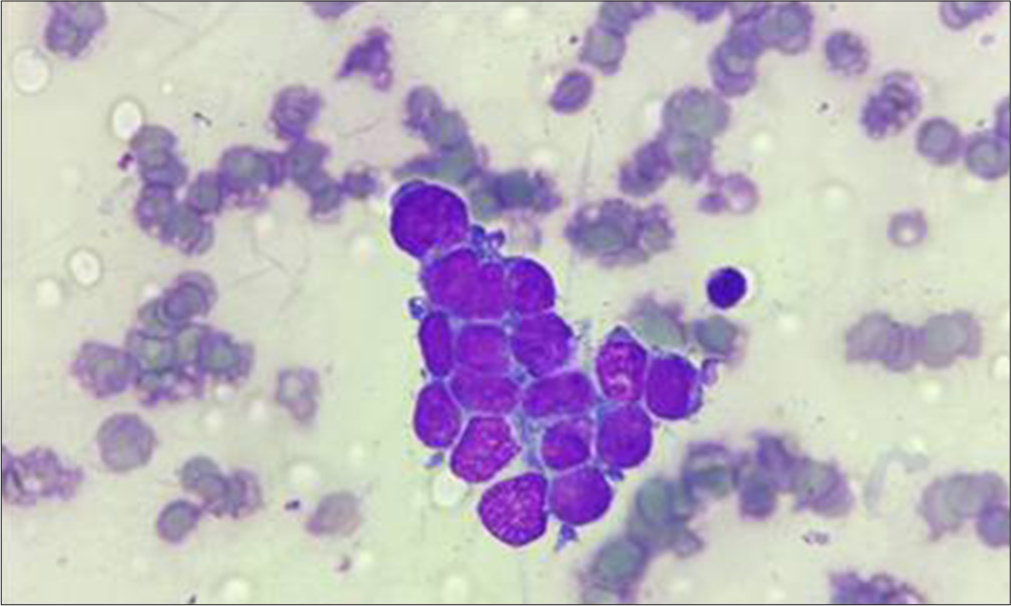
- Bone marrow aspirate smears show clusters of round blue cells. Hematoxylin and eosin (200x)
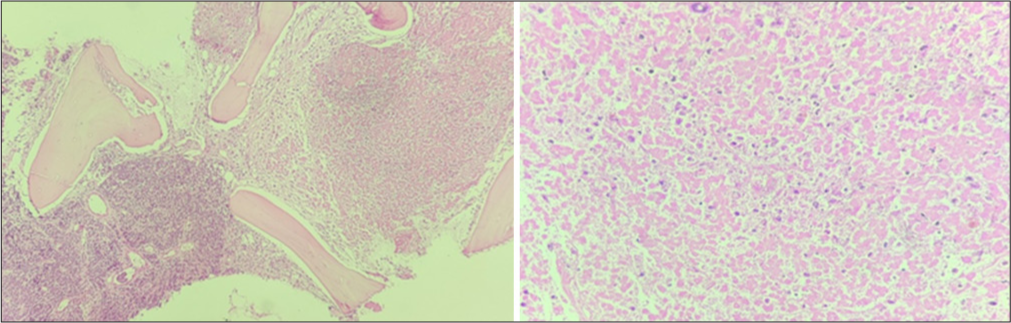
- Bone marrow biopsy with area of involvement by abnormal cells and large area of necrosis.
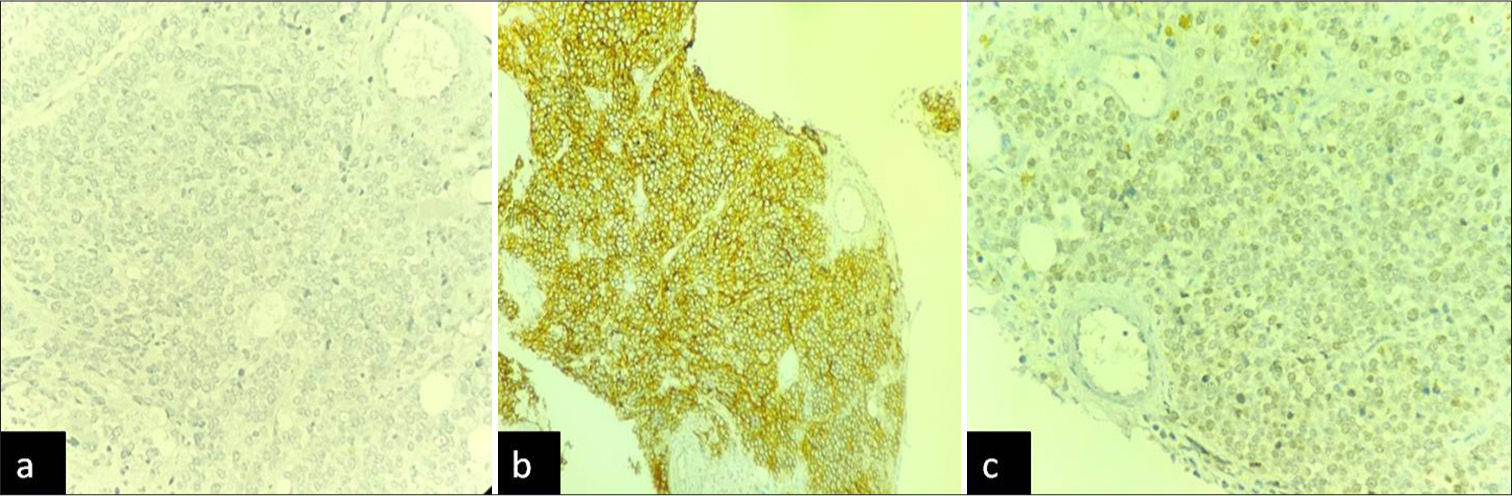
- (a) CD45 immunostain negative in abnormal cells. (b) CD99 immunostain strong positive. (c) NKX 2.2 focal positivity.
DISCUSSION
Ewing sarcoma generally arises in young adults mainly in their mid-20s, our patient was 29 years old; also, there is slight male predilection. The clinical presentation was acute and aggressive with involvement of spine and the lesion in ribs and the bone marrow was involved. The Ewing sarcoma is aggressive tumor and present most of the metastatic involvement. The most common site is lower extremity especially femur (approximately 45% cases), followed by upper extremity (20%) and then the axial skeleton. The bone marrow biopsy showed characteristic involvement by tumor cells with positivity for CD99 and NKX2.2. Out them NKX2.2 is very specific for Ewing sarcoma. The cytogenetics analysis by karyotype was interesting as well as confusing due to complex translocation between chromosome 9, 21, and 22. In comparison with literature, the translocation most commonly found in Ewing is t(11;22)(q24;q12) (EWS-FLI1 fusion) which is found in 85% cases followed by t(21;22)(q22;q12), resulting in EWSR1-ERG fusion in 5–10% cases. The rare subtypes may have complex translocations involving chromosomes 2q, 7p, and 17q resulting in fusion of EWS gene with FEV, ETV1, and ETV4, respectively. In addition, we found trisomy 4 and trisomy 8 in our case. The gain or loss of chromosome 8 is commonly found in more than 50% cases and especially in classic case of Ewing sarcoma with EWS-FLI1.[8] Additional chromosomal abnormalities documented by various studies include gain of chromosome (2, 12, and 20), unbalanced rearrangements with chromosome 1q and loss of 16q were also documented and some studies.[9] None of the studies have mentioned about gain of chromosome 4.
The bad prognostic markers for Ewing’s include traditionally the metastatic status along with the site of tumor, tumor burden, and age of the patient. However, the chromosomal status as well as mutation or genetic re-arrangements needs to be explored for the further prognostic stratification.[10] In our case, axial skeleton was involved along with bone marrow metastasis. Hence, the stage 4 disease was considered and patient was advised best supportive care.
CONCLUSION
The multimodality approach and prompt diagnosis of Ewing sarcoma is important considering the best possible prognosis and therapeutic as well as conservative management available. In addition, bone marrow studies and clinical and cytogenetic correlation can provide newer targeted therapies in Ewing sarcoma if monitored and worked up thoroughly.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Ewing’s sarcoma In: Huvos AG, ed. Bone tumors: Diagnosis, treatment and prognosis (2nd ed). Philadelphia, PA: Sanders; 1991. p. :523-52.
- [Google Scholar]
- The Ewing family of tumors. Ewing's sarcoma and primitive neuroectodermal tumors. Pediatr Clin North Am. 1997;44:991-1104.
- [CrossRef] [PubMed] [Google Scholar]
- Ewing's sarcoma: Diagnostic, prognostic, and therapeutic implications of molecular abnormalities. J Clin Pathol. 2003;56:96-102.
- [CrossRef] [PubMed] [Google Scholar]
- Promiscuous partnerships in Ewing's sarcoma. Cancer Genet. 2011;204:351-65.
- [CrossRef] [PubMed] [Google Scholar]
- Ewing sarcoma with ERG gene rearrangements: A molecular study focusing on the prevalence of FUS-ERG and common pitfalls in detecting EWSR1-ERG fusions by FISH. Genes Chromosomes Cancer. 2016;55:340-9.
- [CrossRef] [PubMed] [Google Scholar]
- Ewing sarcoma and the history of similar and possibly related small round cell tumors: From whence have we come and where are we going? Adv Anat Pathol. 2018;25:314-26.
- [CrossRef] [PubMed] [Google Scholar]
- Management of Ewing sarcoma family of tumors: Current scenario and unmet need. World J Orthop. 2016;7:527-38.
- [CrossRef] [PubMed] [Google Scholar]
- RAD21 is a driver of chromosome 8 gain in Ewing sarcoma to mitigate replication stress. Genes Dev. 2021;35:556-72.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical correlations of genetic changes by comparative genomic hybridization in Ewing sarcoma and related tumors. Cancer Genet Cytogenet. 1999;114:35-41.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic impact of chromosomal aberrations in Ewing tumours. Br J Cancer. 2002;86:1763-9.
- [CrossRef] [PubMed] [Google Scholar]






