Translate this page into:
RNA-based therapeutics for the treatment of blood disorders: A review and an overview
*Corresponding author: Kaustav Ghosh, Department of Hematology, Nilratan Sircar Medical College and Hospital, Kolkata, West Bengal, India. ghoshrony94@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mandal PK, Ghosh K. RNA-based therapeutics for the treatment of blood disorders: A review and an overview. J Hematol Allied Sci. 2025;5:47-53. doi: 10.25259/JHAS_4_2025
Abstract
RNA therapeutics involves the use of RNA-based molecules to influence biological and molecular processes to treat specific diseases or alleviate symptoms. This includes therapies such as antisense oligonucleotides (ASO), small interfering RNA (siRNA), microRNA, and aptamers. Fitusiran, an siRNA therapeutic targeting antithrombin, is used for bleeding prevention in hemophilia A and B. RNA interference and splice-switching oligonucleotides are being developed for transfusion-dependent thalassemia with the goal of reducing α-globin synthesis and boosting γ-globin expression. Givosiran, an food and drug administration (FDA)-approved siRNA, treats acute hepatic porphyria. In addition, siRNAs such as Patisiran and Vutrisiran, along with the ASO Inotersen, are FDA-approved for transthyretin amyloidosis. Ongoing research aims to address conditions such as acute myeloid leukemia, myelodysplastic syndromes, cutaneous T-cell lymphoma, chronic lymphocytic leukemia, diffuse large B-cell lymphoma, and multiple myeloma. This review seeks to compile the latest developments in this rapidly advancing area of hematology.
Keywords
Acute hepatic porphyria
Hemophilia A and B
RNA therapeutics
Thalassemia
INTRODUCTION
Nucleic acids play a key role in biological functions and hold significant promise for therapeutic applications. RNA-based therapies use RNA molecules to regulate biological processes, aiming to treat particular diseases or ease symptoms. Different forms of RNA therapeutics offer distinct benefits that are difficult to achieve with other types of medications.[1]
TYPES OF RNA THERAPY
Antisense oligonucleotide (ASO)
ASOs are short, synthetic nucleic acid polymers, usually 18–30 nucleotides long, that bind to specific RNA sequences through Watson-Crick base pairing. Due to their phosphodiester backbone, these molecules are prone to degradation by nucleases and have difficulty crossing the plasma membrane. As a result, considerable research has focused on improving their stability and cellular uptake through various chemical modifications.[2]
ASOs promote the degradation of target messenger RNA (mRNA) by binding to its specific sequence. Once ASOs bind to the mRNA, the RNase H endonuclease recognizes the heteroduplex and breaks down the mRNA, leading to a reduction in gene expression. In addition, ASOs can alter the splicing of pre-mRNAs by blocking the binding of splicing factors to important cis-elements, such as splice sites, exonic splicing enhancers, and intronic splicing silencers. This approach is widely used in therapeutic strategies.[3]
Small interfering RNA (siRNA)
siRNAs are non-coding RNAs that target and degrade mRNA from specific genes. Exogenous double-stranded precursor siRNAs are introduced into the cell, where the Dicer enzyme processes them into 20–25 base pair fragments. These fragments are then incorporated into the Argonaute (Ago) protein, with the sense strand being discarded. The remaining antisense strand, along with Ago, forms the RNA-induced silencing complex (RISC), which then locates and binds to the target mRNA, resulting in its degradation. Mechanism of gene silencing by siRNA is outlined in Figure 1.[4]
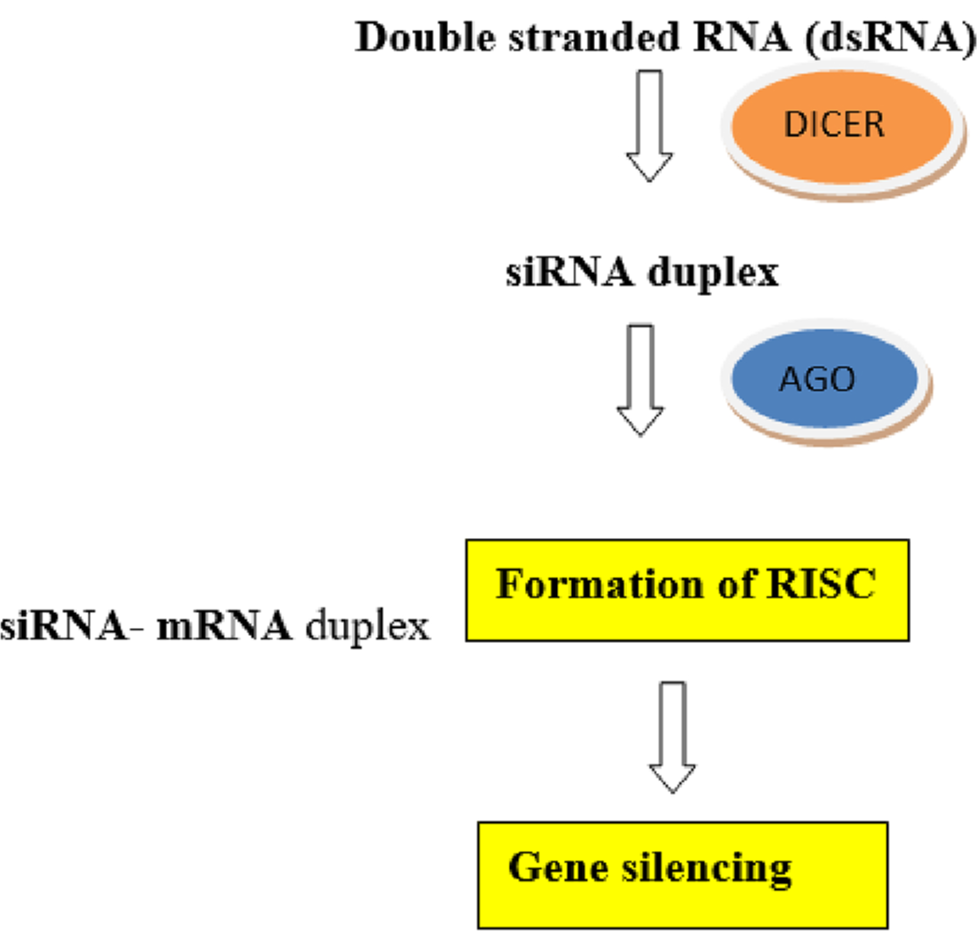
- Mechanism of gene silencing by small interfering RNA.
SiRNA therapy
RNA interference (RNAi), also referred to as post-transcriptional gene silencing, is an evolutionarily conserved process that lowers gene expression by targeting and degrading specific mRNA molecules with RNA molecules. In this RNAi pathway, siRNA plays a crucial role. Gene expression is suppressed when short RNA molecules, such as microRNAs (miRNAs) or siRNAs, bind to and deactivate endogenous mRNA. The Nobel Prize in Physiology or Medicine 2006 was awarded jointly to Boyce S, Rangarajan S for their discovery of RNAi.[5]
miRNA
miRNA is a short, non-coding RNA molecule (19–25 nucleotides) that degrades mRNA in a manner similar to siRNA. miRNA genes produce single-stranded RNA transcripts that form a hairpin structure. Inside the nucleus, these transcripts are initially processed by the enzyme Drosha, which has an RNase III domain. Following this, Exportin5 transports the processed miRNA to the cytoplasm, where it undergoes further processing by Dicer. The resulting single-stranded miRNAs then bind to the Ago protein to form the RISC.[6]
Table 1 summarizes the differences between siRNA and miRNA.
| siRNA | miRNA | |
|---|---|---|
| Before Dicer processing | Double-stranded RNA that contains 30 to over 100 nucleotides | Pre-miRNA that contains 70–100 nucleotides with interspersed mismatches and hairpin structure |
| Structure | 21–23 nucleotide RNA duplex with 2 nucleotides 3’overhang | 19–25 nucleotide RNA duplex with 2 nucleotides 3’overhang |
| Complementary | Fully complementary to mRNA | Partially complementary to mRNA, typically targeting the 3’ untranslated region of mRNA |
| mRNA target | One | Multiple |
| Mechanism of gene regulation | Endonucleolytic cleavage of mRNA | Translational repression Degradation of mRNA Endonucleolytic cleavage of mRNA |
| Clinical applications | Therapeutic agent | Drug target Therapeutic agent Diagnostic and biomarker tool |
siRNA: small interfering RNA, miRNA: MicroRNA, pre-miRNA: Precursor miRNA, mRNA: Messenger RNA
Aptamers
Aptamers are short, single-stranded nucleic acids that bind specifically to proteins, with their function dependent on their three-dimensional structures. They can act in various capacities, such as agonists, antagonists, bispecific aptamers, or drug carriers. Although their functions are similar to those of antibodies, RNA aptamers are smaller in size and can more easily cross cell membranes.[7]
RNA therapy in hemophilia
Unmet needs and challenges with current treatment options[8]
Limited protection from current prophylaxis: Regular FVIII replacement therapy, administered 2–3 times a week, provides inconsistent protection due to the short half-life of the infused FVIII.
High frequency of anti-FVIII antibodies: Approximately 25–30% of patients with severe hemophilia A and 3–13% with moderate or mild hemophilia develop anti-FVIII antibodies. More than 95% of these inhibitors develop within the first 50 days of treatment.[9]
Breakthrough bleeding despite prophylaxis: Patients with FVIII inhibitors are still at risk for significant and potentially life-threatening bleeds, even when receiving the recommended doses of on-demand or prophylactic bypassing agents.
Difficulties with venous access: All current hemophilia treatments involving clotting factor concentrates (CFCs) require intravenous infusion, which poses a challenge for patients.
Rigorous immune tolerance induction (ITI) regimen: ITI therapy requires high doses of CFCs and frequent infusions every 1–2 days, potentially lasting 18– 24 months, with success rates of about 60–70% in individuals with hemophilia.
Hence, eliminating the inhibitors of coagulation by employing non-factor replacement therapies that either block or remove anticoagulants has demonstrated promise in restoring hemostasis in hemophilia without the need to replace the missing clotting factor.
Fitusiran:[5] It is a siRNA-based treatment developed to prevent bleeding in hemophilia A and B. This synthetic siRNA is chemically attached to a triantennary N-acetylgalactosamine (GalNAc) ligand. Th GalNAc-siRNA complex specifically targets and reduces the mRNA of antithrombin, leading to a decrease in antithrombin production. As a result, there is enhanced inhibition of thrombin and other serine proteases, including factors Xa, IXa, and XIIa.
In the phase 1 dose escalation study by Pasi et al.,[10] the safety and tolerability of subcutaneous fitusiran were assessed in four healthy volunteers and 25 adults with moderate to severe hemophilia A and B who did not have inhibitors. The healthy volunteers received a single subcutaneous dose of fitusiran at 0.03 mg/kg of body weight. The hemophilia participants received three doses of fitusiran, either weekly (at doses of 0.015, 0.045, or 0.075 mg/kg) or monthly (at doses of 0.225, 0.45, 0.9, or 1.8 mg/kg, or a fixed dose of 80 mg).
Peak plasma levels of fitusiran were observed 2–6 h after administration. In patients with hemophilia, plasma levels of fitusiran rose while antithrombin levels decreased in a dose-dependent manner. The reduction in antithrombin was associated with increased thrombin generation, with similar outcomes in both hemophilia A and B patients. The pharmacological effect was prolonged, with antithrombin recovery occurring at a median rate of 10–15% per month after discontinuing fitusiran.
Fitusiran was generally well tolerated, with injection site reactions and arthralgia being the most common side effects. Transient increases in liver aminotransferases were observed in 36% of participants, but no patients developed anti-fitusiran antibodies. Monthly fitusiran dosing was well tolerated, lowered antithrombin levels from baseline, and improved thrombin generation. It was concluded that monthly treatment with fitusiran could help reduce bleeding episodes. Ongoing phase III trials for fitusiran are ATLAS-PEDS, ATLAS-OLE, ATLAS-PPX, ATLAS-INH, and ATLAS A/B.[11] Other potential targets for RNAi therapy are protein S and heparin cofactor II.[10]
RNA therapy in thalassemia
In β-thalassemia, the condition arises from a single point mutation or a small deletion in the β-globin gene. The primary goal of treatment is to achieve a balanced ratio of α to β chains, which helps minimize the surplus of free α-chains.[12]
Several nucleic acid-based approaches could serve as precision medicine options for β-thalassemia, including:
Decreasing α-globin expression-[13] To achieve a balanced α/β-globin ratio, it is essential to reduce α-globin mRNA expression by 25–50%, while maintaining stable levels of β-globin mRNA. siRNA or miRNA form complexes with the RISC, which then cleaves the mRNA with the help of RNAse, resulting in reduced α-globin expression.
Modifying β-globin pre-mRNA splicing to correct mutations-[14] A major molecular mechanism behind the defect in β-globin gene expression is mutation-induced abnormal splicing (e.g., mutations such as IVS1–5, IVS1–6, and IVS1–110 in intron 1), which activates non-canonical splice sites and disrupts normal splicing pathways. Such splicing errors also lead to the formation of abnormal hemoglobins, like HbE. The activation of these atypical splice sites causes incorrect RNA splicing, resulting in the retention of intron fragments and the production of non-functional β-globin chains. Short synthetic oligonucleotides (15–25 nucleotides) targeting specific pre-mRNA sequences can alter the recognition of splice sites by the spliceosome. This modification influences the splicing of the targeted transcript, which is why these oligonucleotides are called splice-switching oligonucleotides (SSOs). Mechanisms by which SSOs modulate RNA splicing: Exon skipping, Exon retention and Restoration of correct splicing.
Reactivating γ-globin expression-[15] Elevated levels of HbF can help reduce the clinical severity of β-thalassemia, especially in cases co-inherited with HPFH or the Xmnl1-HBG2 polymorphism. The increased γ-globin chains interact with surplus α-globin chains, improving the balance between the globin chains and mitigating the disease’s severity. The expression of the γ-globin gene is controlled by three transcription factors: BCL11A, KLF1, and MYB, which suppress its expression after birth. siRNA can be utilized to inhibit BCL11A production, effectively boosting HbF levels.
RNA therapy in porphyria
Acute hepatic porphyria refers to a collection of inherited metabolic disorders that impair heme production in the liver. Enzyme deficiencies cause a buildup of neurotoxic or phototoxic heme precursors, resulting in symptoms associated with neurovisceral problems or sensitivity to light.[16]
Givosiran
It is an siRNA targeting 5-aminolevulinic acid synthase (ALAS1), which is covalently linked to a ligand containing three GalNAc residues to enhance delivery to hepatocytes.[17] The aim is to reduce ALAS1 activity and lower the levels of toxic heme intermediates, primarily 5-aminolevulinic acid and porphobilinogen.
In the phase III trial by Balwani et al.,[18] 94 patients with acute hepatic porphyria were randomly assigned to receive either subcutaneous givosiran (2.5 mg/kg body weight) or a placebo once a month for 6 months. The primary endpoint was the annualized rate of composite porphyria attacks in patients with acute intermittent porphyria, the most common form of acute hepatic porphyria.
The mean annualized attack rate was 3.2 in the givosiran group, compared to 12.5 in the placebo group, demonstrating a 74% reduction in attacks in the givosiran group (P < 0.001). In patients with acute intermittent porphyria, givosiran led to lower urinary levels of ALA and porphobilinogen, fewer days requiring hemin treatment, and improved daily pain scores compared to the placebo.
Common adverse events in the givosiran group included elevated serum aminotransferase levels, changes in serum creatinine levels and estimated glomerular filtration rate, and injection-site reactions.
Gene-based therapies currently under development show considerable promise for improving patient care. New treatments being explored, such as pharmacological chaperones, aim to stabilize or activate wild-type hydroxymethylbilane synthase and its mutants associated with acute intermittent porphyria. These therapies could extend the enzyme’s half-life, improve its function, and potentially offer preventative treatment for the disease.
RNA therapy in amyloidosis
Transthyretin (TTR) amyloidosis occurs when the TTR protein misfolds. TTR is a tetramer produced by the liver and functions as a transport protein for thyroxine and retinol. There are two types of TTR amyloidosis.[19]
Wild-type ATTR, which occurs secondary to an acquired pathogenic process associated with aging.
-
Hereditary ATTR (hATTR or ATTRv), which occurs secondary to an inherited TTR-gene mutation.
SiRNA therapy-Patisiran was the first siRNA developed and received approval from the United States Food and Drug Administration (FDA) and the European Commission in 2018 for treating ATTRv-PN. It is delivered as a lipid nanoparticle, which separates the double-stranded siRNA into single strands. Once released into the hepatocyte cytoplasm, the single-stranded siRNA binds to complementary mRNA, activating the Ago slicer protein. This results in the degradation of the mRNA and inhibits TTR synthesis.[20]
In the APOLLO phase 3 trial,[21] done on 225 patients, patisiran (at a dose of 0.3 mg/kg for those under 100 kg and 30 mg for those over 100 kg) demonstrated a significant improvement from baseline in the modified Neuropathy Impairment Score + 7 in the treatment group compared to the placebo group. In addition, it showed improvements in the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy score and gait speed.
Other siRNA therapy drugs in development include revusiran and vutrisiran.[22,23]
ASO- Inotersen: It is a 2’-O-methoxyethyl-modified ASO that targets the 3’ untranslated region of complementary mRNA, leading to its degradation through ribonuclease H1-mediated action. It was the first ASO to be developed and approved by the FDA and EMA in 2018 for treating ATTRv-PN.[24]
In the phase III NEURO-TTR[24] trial, in adults with stage 1 (ambulatory) or stage 2 (ambulatory with assistance), hereditary TTR amyloidosis with polyneuropathy was randomly assigned in a 2:1 ratio to receive weekly subcutaneous injections of inotersen (300 mg) or placebo. The study concluded that inotersen improved the progression of neurological disease and quality of life in patients with hereditary TTR amyloidosis. Thrombocytopenia and glomerulonephritis were managed through increased monitoring.[25]
Eplontersen is another ASO for the treatment of hTTR amyloidosis-related polyneuropathy, with ongoing evaluation.[26]
Future directions
Potential of RNA therapy lies in its ability to precisely target and regulate the expression of genes involved in the development and progression of these cancers. It works by gene silencing, modulating gene expression, overcoming drug resistance, and targeting immune checkpoints and leukemic stem cells along with providing personalized treatment. As shown in Table 2 below, RNA therapeutics are being evaluated in a number of other hematological disorders including acute myeloid leukemia (AML), diffuse large B-cell lymphoma (DLBCL), myelodysplastic syndromes (MDS), cutaneous T-cell lymphoma (CTCL), chronic lymphocytic leukemia (CLL), and multiple myeloma (MM).[1] Table 3 shows FDA approved RNA therapeutics in other disorders.[2]
| NCT number phase disease target |
|---|
| NCT02781883 Phase 2 AML GRB2 |
| NCT02549651 Phase 1 DLBCL STAT3 |
| NCT02243124 Phase 1 MDS TP53 |
| NCT03713320 Phase 2 CTCL miR-155 |
| NCT01486797 Phase 2 CLL CXCL12 |
| NCT01521533 Phase 2 MM CXCL12 |
AML: Acute myeloid leukemia, DLBCL: Diffuse large B-cell lymphoma, MDS: Myelodysplastic syndromes, CTCL: Cutaneous T-cell lymphoma, CLL: Chronic lymphocytic leukemia, MM: Multiple myeloma
| Drug name | FDA approved | Indications | Drug modality | Mechanisms | Targets |
|---|---|---|---|---|---|
| Fomivirsen | 1998 | CMV retinitis | ASO | Translation block | CMV protein IE2 |
| Pegaptanib | 2004 | Neovascular age-related macular degeneration | Aptamer | Binding and blocking | Heparin-binding domain of VEGF-165 |
| Mipomersen | 2013 | Homozygous familial hypercholesterolemia | ASO | RNase H degradation | Apolipoprotein B100 |
| Eteplirsen | 2016 | Duchenne muscular dystrophy | ASO | Splicing modulation | Exon 51 of DMD |
| Nusinersen | 2016 | Spinal muscular atrophy | ASO | Splicing modulation | Exon 7 of SMN2 |
| Defibrotide | 2016 | Veno-occlusive disease in liver | Aptamer | Binding and blocking | Adenosine A1/A2 receptor |
| Milasen | 2018 | Mila Makovec’s CLN7 gene associated with Batten disease | ASO | Splicing modulation | CLN7 |
| Golodirsen | 2019 | Duchenne muscular dystrophy | ASO | Splicing modulation | Exon 53 of DMD |
| Viltolarsen | 2020 | Duchenne muscular dystrophy | ASO | Splicing modulation | Exon 53 of DMD |
| Casimersen | 2021 | Duchenne muscular dystrophy | ASO | Splicing modulation | Exon 45 of DMD |
FDA: Food and drug administration, ASO: Antisense oligonucleotides, CMV: Cytomegalovirus
Advantages of RNA therapy
Targeting the untargetable, treating the untreatable
Quick production
Patient customized therapy.
Disadvantages of RNA therapy
Poor bioavailability
Short lived response
Off target effects.
Status of RNA therapeutics in India
Although RNA therapeutics are gaining considerable global attention, their development in India is still in the early phases, with challenges such as a lack of effective delivery systems, limited research on silencing agents, and difficulties in translating nucleic acid-based therapies into clinical trials. However, there is increasing interest and efforts to build domestic capabilities in this field, including collaborations with international research institutions to address these challenges and advance RNA therapeutics in India.[27]
CONCLUSION
RNA therapeutics represents a therapy with the use of RNA-based molecules to modulate molecular and biological processes to cure a specific disease or improve symptoms
It includes ASO, siRNA, miRNA, and Aptamers
Fitusiran is a RNAi therapeutic targeting antithrombin for prophylaxis of bleeding in hemophilia A and B
RNAi and SSO are in development for transfusion-dependent thalassemia aimed at reducing α-globin synthesis and reactivating γ-globin expression
Givosiran is FDA approved siRNA for acute hepatic porphyria
Patisiran, vutrisiran (siRNA), and inotersen (ASO) are FDA approved for TTR amyloidosis
Future developments in AML, MDS, CTCL, CLL, DLBCL, and MM are in process.
Acknowledgment
We would like to show our immense gratitude to the researchers and authors who encouraged us to read the relevant topics in depth and inspired to write this systematic review.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Recent advances in oligonucleotide therapeutics in oncology. Int J Mol Sci. 2021;22:3295.
- [CrossRef] [PubMed] [Google Scholar]
- RNA-based therapeutics: An overview and prospectus. Cell Death Dis. 2022;13:644.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509-24.
- [CrossRef] [PubMed] [Google Scholar]
- RNAi for the treatment of people with hemophilia: Current evidence and patient selection. J Blood Med. 2023;14:317-27.
- [CrossRef] [PubMed] [Google Scholar]
- siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucl Acids. 2015;4:e252.
- [CrossRef] [PubMed] [Google Scholar]
- Achieving and maintaining an optimal trough level for prophylaxis in haemophilia: The past, the present and the future. Blood Transfus. 2014;12:314.
- [Google Scholar]
- Establishing the prevalence and prevalence at birth of hemophilia in males: A meta-analytic approach using national registries. Ann Intern Med. 2019;171:540-6.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med. 2017;377:819-28.
- [CrossRef] [PubMed] [Google Scholar]
- Paradigm shift for the treatment of hereditary haemophilia: towards precision medicine. Blood Rev. 2020;39:100618.
- [CrossRef] [PubMed] [Google Scholar]
- Nucleic acid therapy for β-thalassemia. Biologics. 2020;14:95-105.
- [CrossRef] [PubMed] [Google Scholar]
- siRNA-mediated reduction of β-globin results in phenotypic improvements in α-thalassemic cells. Haematologica. 2008;93:1238-42.
- [CrossRef] [PubMed] [Google Scholar]
- Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res. 2016;44:6549-63.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of γ/β globin gene switching in CD 34+ derived erythroid cells by BCL11A RNA silencing. Indian J Hematol Blood Transfus. 2019;35:758-64.
- [CrossRef] [PubMed] [Google Scholar]
- Heme biosynthesis and the porphyrias. Mol Genet Metab. 2019;128:164-77.
- [CrossRef] [PubMed] [Google Scholar]
- RNAi therapy with givosiran significantly reduces attack rates in acute intermittent porphyria. J Intern Med. 2022;291:593-610.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N Engl J Med. 2020;382:2289-301.
- [CrossRef] [PubMed] [Google Scholar]
- RNA targeting and gene editing strategies for transthyretin amyloidosis. Biodrugs. 2023;37:127-42.
- [CrossRef] [PubMed] [Google Scholar]
- A review of patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol Ther. 2020;9:301-15.
- [CrossRef] [PubMed] [Google Scholar]
- Trial design and rationale for APOLLO, a Phase 3, placebo-controlled study of Patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy. BMC Neurol. 2017;17:181.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical proof of concept for a novel hepatocyte-targeting GalNAc-siRNA conjugate. Mol Ther. 2017;25:71-8.
- [CrossRef] [PubMed] [Google Scholar]
- Single-dose pharmacokinetics and pharmacodynamics of transthyretin targeting N-acetylgalactosamine-small interfering ribonucleic acid conjugate, vutrisiran, in healthy subjects. Clin Pharmacol Ther. 2021;109:372-82.
- [CrossRef] [PubMed] [Google Scholar]
- Suppressing transthyretin production in mice, monkeys and humans using 2nd-Generation antisense oligonucleotides. Amyloid. 2016;23:148-57.
- [CrossRef] [PubMed] [Google Scholar]
- Early data on long-term efficacy and safety of inotersen in patients with hereditary transthyretin amyloidosis: A 2-year update from the open-label extension of the NEURO-TTR trial. Eur J Neurol. 2020;27:1374-81.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of eplontersen on cardiac structure and function in patients with hereditary transthyretin amyloidosis. J Card Fail. 2024;30:973-80.
- [CrossRef] [PubMed] [Google Scholar]
- Current status in clinical advancement of RNA therapeutics-Correspondence. Int J Surg. 2022;108:106996.
- [CrossRef] [PubMed] [Google Scholar]







