Translate this page into:
t (1;12) (p31; q13) cytogenetic abnormality in a patient of idiopathic hypereosinophilic syndrome: A case report
*Corresponding author: Arjun Kachhwaha, Department of Hematology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India. drarjunk95@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kachhwaha A, Satadeve P, Gupta A, Nath UK. t (1;12) (p31; q13) cytogenetic abnormality in a patient of idiopathic hypereosinophilic syndrome: A case report. J Hematol Allied Sci. 2024;4:83-6. doi: 10.25259/JHAS_30_2024
Abstract
Hypereosinophic syndrome (HES) is a heterogenous group of disorders comprising various etiology. Idiopathic HES is diagnosis of exclusion after morphological, radiological and molecular investigations. Cytogenetic analysis remains an important diagnostic test available in HES. The patient reported here presented with hyper eosinophilia with cardiac, gastrointestinal and pulmonary organ involvement. Chromosomal abnormality of t (1;12) (p31; q13) was found in conventional karyotyping (unstimulated culture, GTG banding) on bone marrow sample. Patient improved after short course of corticosteroid and imatinib mesylate a tyrosine kinase inhibitor (TKI).
Keywords
Hypereosinophic syndrome (HES)
Cytogenetic abnormality
tyrosine kinase inhibitor (TKI)
INTRODUCTION
Hypereosinophilia is defined by an absolute eosinophil count (AEC) of more than ≥1.5 × 109/L.[1,2] Eosinophils and their derived mediators cause various organ damage ranging from asymptomatic to life-threatening.[1-4] Predominant complications associated with hypereosinophilic syndrome (HES) are fibrotic and thrombotic.[1] There are many subtypes under HES, and a panel of investigations which are required to prove clonality. Next-generation sequencing (NGS) and karyotyping remain important investigations in these patients,[4] and here, we are presenting a case with a rare cytogenetic abnormality detected on conventional karyotyping.
CASE REPORT
Presentation
A 54-year-old female known case of hypertension, type 2 diabetes mellitus, and former smoker was presented with complaints of recurrent episodes of nausea, vomiting, generalized weakness, and exertion dyspnea with orthopnea (The New York Heart Association IV) with increased frequency of loose stools (semisolid, non-foul smell, non-blood stained, without mucous) for past 1 and 1/2 month. On general examination, pallor was present, and there was no peripheral lymphadenopathy palpable on examination. Asymmetric left lower limb edema was noted up to the mid-thigh region. Systemic examination was unremarkable except chest auscultation that revealed bilateral coarse crepitation with wheeze predominantly in lower areas. There was no organomegaly. The patient was outside treated with antiparasitic agents, nebulization and antibiotics along with other supportive care without any symptomatic benefits and referred to our institution.
Management
On admission, all relevant hematological and biochemical investigations were done [Table 1]. A hemogram and peripheral smear revealed an AEC of 235610/μL. Kidney function and liver function tests were within normal limits. Cardiac troponin I was normal. The patient was started methylprednisolone pulse therapy 1000 mg once daily intravenously for 3 consecutive days, followed by oral prednisolone 1 mg/kg along with cytoreductive therapy with oral hydroxyurea, considering significantly increased eosinophil counts. Peripheral blood breakpoint cluster region: ABL proto-oncogene 1 (BCRABL1) by real-time polymerase chain reaction was negative. Ultrasound sonography venous color Doppler was done in view of asymmetrical left lower limb swelling, revealing acute thrombosis of the left common femoral vein and saphenofemoral vein. The patient was started on subcutaneous enoxaparin injection followed by oral apixaban on discharge. Contrast-enhanced computerized tomography thorax, abdomen, and pelvis, along with computed tomography pulmonary angiogram, were done to rule out underlying occult malignancy and to look for evidence of pulmonary embolism with background deep vein thrombosis, which showed mild bilateral pleural effusion and mild ascites. 2D transthoracic echocardiogram was suggestive of the presence of regional wall motion abnormalities (RWMA), moderate eccentric mitral regurgitation, and grade 1 left ventricular diastolic dysfunction (LVDD) with ejection fraction (EF) of 45%. The stool routine for parasites examination and occult blood was negative, and upper and lower gastrointestinal (GI) endoscopy was planned in view of suspected GI involvement by eosinophilia. Later patient refused the same. Bone marrow examination revealed cellular marrow with a marked increase in eosinophilic precursors with evidence of dyspoiesis in eosinophilic and megakaryocytic lineages. Conventional karyotyping [Figure 1] by unstimulated culture and GTG banding showed t (1;12) (p31; q13). The NGS panel for hypereosinophilia syndrome did not detect any clinically relevant gene fusions. The patient was started on oral imatinib 100 mg once daily, along with oral steroid therapy.[5,6] The dose of imatinib was increased on the follow-up visit based on eosinophil count, and currently, the patient is on oral imatinib 400 mg once daily dose. The patient in the last follow is symptomatically better, and the latest eosinophil count is 570/μL.
| Hemoglobin | 10.5 g/dL |
| Total leucocyte counts | 235610/μL |
| AEC | 212400/μL |
| Platelet counts | 351000/μL |
| Peripheral blood smear | RBCs: Normocytic normochromic to few microcytic hypochromic RBCs. WBCs: Marked leucocytosis with severe eosinophilia (AEC 2,12,400/μL), 90% eosinophils in the peripheral blood. Many of them are monolobulated, bilobulated, and multilobulated, and many show hypo granulation. DLC: Eosinophils – 90%, monocytes – 04%, lymphocytes – 05%, neutrophils – 01%, Platelets: Adequate. No hemoparasites were seen. |
| Liver function test | Total bilirubin: 0.64 mg/dL (0.3–1.2) Direct bilirubin: 0.05 mg/dL (0–0.2) SGPT: 34.0 U/L (0–35) SGOT: 28.0 U/L (0–35) ALP: 263.0 U/L (30–120) GGT: 54.0 U/L (0–38) Serum total protein: 6.9 g/dL (6.6–8.3) Serum albumin: 2.9 g/dL (3.5–5.2) Serum globulin: 4.0 g/dL (2.5–3.2) |
| Kidney function test | Blood urea: 27.0 mg/dL (17–43) Serum creatinine: 0.60 mg/dL (0.55–1.02) Serum Na+: 136.0 mmol/L (136–146) Serum K+: 3.89 mmol/L (3.5–5.1) Serum Cl: 98.0 mmol/L (101–109) Serum total calcium: 9.66 mg/dL (8.8–10.6) Serum uric acid: 4.0 mg/dL (2.6–6) Phosphorus: 1.4 mg/dL (2.5–4.5) |
| Thyroid profile | TSH: 0.70 μIU/mL (0.13–6.33) T4: 6.33 μg/dL (4.5–12.6) |
RBCs: Red blood cells, WBCs: White blood cells, AEC: Absolute eosinophil count, TSH: Thyroid-stimulating hormone, SGPT: Serum glutamic pyruvic transaminase, SGOT: Serum glutamic oxaloacetic transaminase, ALP: Alkaline phosphatase, GGT: Gamma-glutamyl transferase, Cl: Chloride, Na: Sodium, K: Potassium, T4: Thyroxine, DLC: Differential leucocyte count
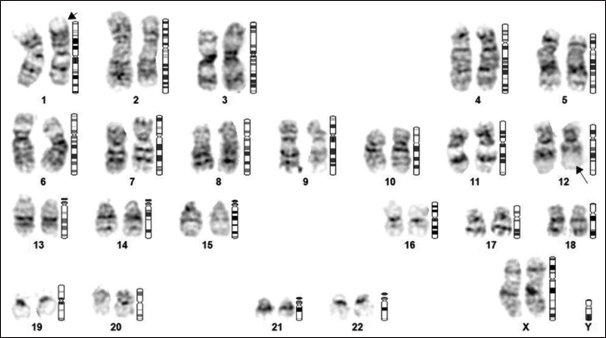
- Chromosome analysis revealed 46, XX with the presence of balanced translocation between the short arm of chromosome #1 (arrow) and the long arm of chromosome #12 (arrow), between the regions 1p31 and 12q13, respectively, found in all the metaphases studied.
DISCUSSION
HES is a heterogeneous group of disorders, and molecular and genetic study is required to reach a particular diagnosis. HES is a diagnosis of exclusion.[7] NGS and karyotyping or fluorescence in situ hybridization (FISH) remains a very helpful diagnostic modality.[8] There are various chromosomal abnormalities described with regard to HES.
HES can involve various organ systems with varying severity which needs to be evaluated and managed.[9]
In the present case, the chromosomal abnormality detected was t (1;12) (p31; q13), and from my knowledge, it is not mentioned so far in the literature.
CONCLUSION
t (1;12) (p31; q13) chromosomal abnormality in conventional karyotyping in HES is a rare finding. Along with other investigations, including immunophenotyping by flow cytometry and NGS for mutation study, karyotyping and FISH are important and recommended.
Standard of reporting
CARE guidelines and methodology were followed to conduct the study.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy. 2023;78:47-59.
- [CrossRef] [PubMed] [Google Scholar]
- Approach to the patient with suspected hypereosinophilic syndrome. Hematol Am Soc Hematol Educ Program. 2022;2022:47-54.
- [CrossRef] [PubMed] [Google Scholar]
- Cytogenetic and molecular cytogenetic characterization of 6 new cases of idiopathic hypereosinophilic syndrome. Haematologica. 2000;85:486-91.
- [Google Scholar]
- Hypereosinophilic syndromes-An enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. . 2021;49:100809.
- [CrossRef] [PubMed] [Google Scholar]
- Imatinib therapy for hypereosinophilic syndrome and other eosinophilic disorders. . 2003;101:3391-7.
- [CrossRef] [PubMed] [Google Scholar]
- How I treat hypereosinophilic syndromes. Blood. 2015;126:1069-77.
- [CrossRef] [PubMed] [Google Scholar]
- Imatinib mesylate as a novel treatment option for hypereosinophilic syndrome: Two case reports and a comprehensive review of the literature. Ann Hematol. 2006;85:1-16.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow morphology is a strong discriminator between chronic eosinophilic leukemia, not otherwise specified and reactive idiopathic hypereosinophilic syndrome. Haematologica. 2017;102:1352-60.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular pathogenesis and treatment perspectives for hypereosinophilia and hypereosinophilic syndromes. Int J Mol Sci. 2021;22:486.
- [CrossRef] [PubMed] [Google Scholar]







